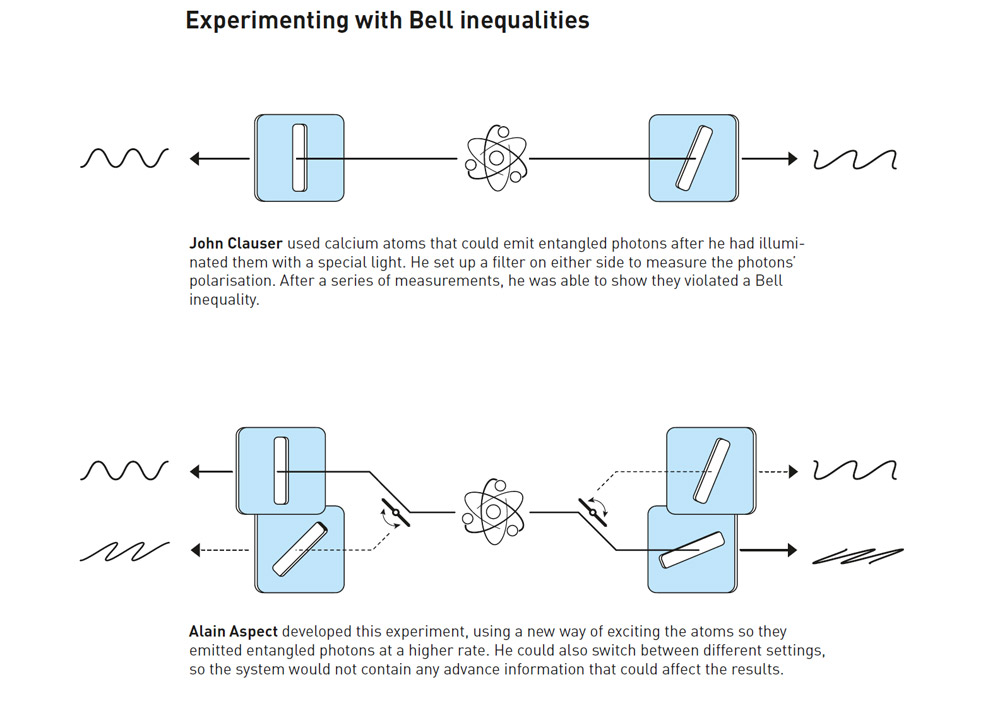
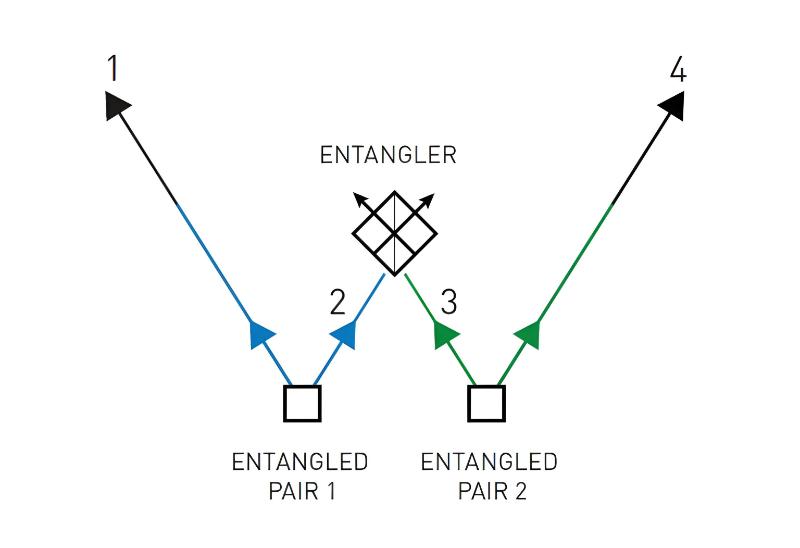
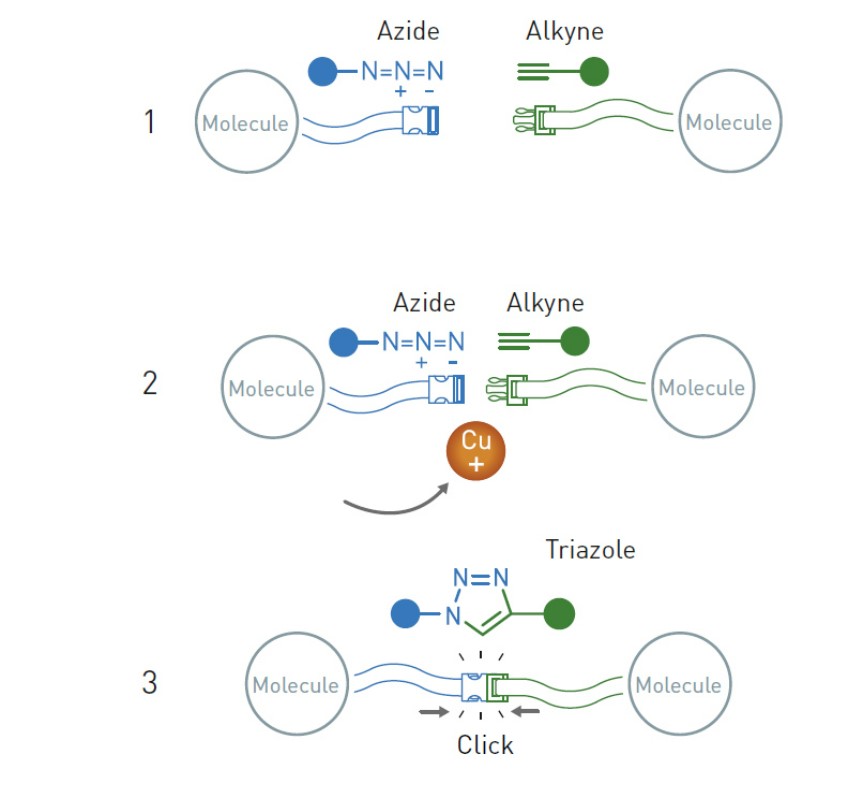
Although it has happened rarely in the history of Nobel prizes for natural sciences, there have been a few instances of laureates whose results were later shown to be incorrect or at least off by a small margin. Some of these caused significant discoveries to be realized far later than they otherwise would have. However, scientific findings are seldom unquestionable. During the course of scientific history, there have been several shifts in the accepted theories, methodologies, and underlying beliefs about what is correct. The full worth of a piece of study can only be judged in hindsight, when everything is crystal clear.
Perhaps more surprising is the fact that just a handful of Nobel laureates in the natural sciences have ever made critical mistakes. Of course, they didn’t always get it right; occasionally, they saw the correct phenomenon but concluded the incorrect idea.
Johannes Fibiger and the Roundworm

The Nobel Prize in Medicine given to the Danish researcher Johannes Fibiger is the most egregious example of a Nobel committee’s misguided decision. The Nobel Committee agreed that Fibiger deserved the prize in 1926 for discovering the worm Spiroptera carcinoma and demonstrating that it caused cancer in rats. The discovery caused quite a stir at a time when researchers were racing to identify the causes of cancer.
Malnourishment, Rather Than Cancer
However, after some time passed, it became obvious that the parasite theory was bunk. His rats did not really acquire cancer, despite the widespread coverage of the disease. As a matter of fact, these were benign tumors of the kind that sometimes manifest themselves in cases of acute vitamin A insufficiency. The reason for this is clear: Fibiger gave his animals a diet of white bread and water, which isn’t exactly species-appropriate.
Because the researcher did not have a non-infected control group close to the rats infected with the nematode, Fibiger did not observe that this may lead to deficient symptoms. In the early 20th century, controlled trials like this were still very uncommon. As a consequence, Fibiger incorrectly diagnosed the tumors as cancer brought on by nematodes.
A New Age Has Begun
Despite the fact that Fibiger’s purported discoveries have been disproven for decades, he is still included on the roll call of medical Nobel laureates. No one has ever acknowledged that Fibiger was incorrect. Instead, there is the original award speech, which includes the following: “Fibiger’s discovery marks the beginning of a new era, a new epoch in the history of cancer… Fibiger was a pioneer in the difficult field of cancer research and will remain so.”
Not until 2004 did the Swedish Karolinska Institute, which had previously been in charge of award selection, admit that Fibiger’s findings were wrong. The nematode that started the whole thing is now known as Gongylonema neoplasticum; the word “carcinoma” has been dropped from the nomenclature.
It’s True, Infections Can Cause Cancer
Although, Fibiger’s idea about parasites was totally off base. Our understanding of the role of viruses and bacteria in cancer development has expanded. Even in a self-experiment conducted in the 1980s, Australian researcher Barry Marshall demonstrated that Helicobacter pylori is the causative agent of stomach ulcers.
The human papillomavirus (HPV) may cause cervical cancer, as identified by German researcher Harald zur Hausen. With this finding, he was awarded the Nobel Prize in Medicine in 2008. Nowadays, this cancer-causing virus is vaccinated against in order to protect young women from getting it in many nations. Epstein-Barr virus (EBV) for nasopharyngeal cancer and Hodgkin’s lymphoma; hepatitis viruses B and C for liver cancer; and human herpesvirus-8 (HHV-8) as a trigger for Kaposi’s sarcoma are just a few of the other viruses that have been shown to promote cancer.
Egas Moniz, Lobotomy Pioneer

Some treatments that have received Nobel Prizes in medicine are very controversial, particularly from an ethical standpoint. Even though they helped some people, they really made many others more miserable when they might have helped them less. This means that, from our vantage point now, they are only partially deserving of a great award.
Healing Through Destruction
Antonio Egas Moniz, a Portuguese physician and neurologist, gained notoriety in 1949 when he claimed to have discovered that lobotomies might alleviate some psychoses. The severing of the frontal lobe’s connections to the thalamus is the primary goal of this operation. Much of the prefrontal cortex and other white matter in the brain are damaged or destroyed.
According to Moniz, the “sticky” nerve connections seen in schizophrenia and other mental disorders are to blame for the patient’s inflexible thoughts and unwavering convictions. Because of this, he thought that cutting them off was the most effective way to help people feel better. As a matter of fact, Moniz’s first patient seemed to be improving as well; she was less disturbed and paranoid than before, yet she was also more lethargic and quiet.
“Every Patient Loses Something”

U.S. psychiatrist Walter Freeman, a leading lobotomy advocate in the 1940s and 1950s, eventually admitted that his patients lost part of their individuality as a result of the operation. “Every patient loses something from this surgery,” he noted; “some spontaneity, some of the radiance, some of the flavor of personality.”
Although Freeman and Moniz before him both agreed that the benefits of alleviating their patients’ psychotic symptoms far exceeded the drawbacks of this approach, Freeman in particular emphasized the importance of preserving patients’ quality of life.
The Nobel Prize Committee agreed, awarding Moniz for this treatment. Bengt Jansson of the Karolinska Institute, who was on the committee, subsequently commented on the matter, writing, “In my opinion there is no doubt that Moniz deserved the Nobel Prize at the time.” Simply said, “Because back then there were no alternatives and the lobotomy actually made life more bearable for some patients and those around them.”
Treatment of Everything With a Lobotomy
The finding by Moniz, and its subsequent public broadcast by Freeman and others, proved catastrophic, however. It caused a flurry of new lobotomies. The “minimally intrusive” treatment that Freeman refined was nearly worshipped as a miracle cure, especially in the United States. He treated people by disrupting their neurological connections by thrusting a sharp device into their brains via their eye sockets, sometimes without anesthetic.
Tens of thousands of patients in the United States alone had this “ice pick” technique, which was touted as a panacea for anything from melancholy and anxiety to schizophrenia and “hysteria.” It was even believed in the 1950s that it might end homophobia, Communism, and violence.
Rosemary Kennedy

The operation wasn’t considered a last measure, but rather an easy way to silence annoying relatives. Rosemary Kennedy, the sister of the late President of the United States, John F. Kennedy, is one of the most well-known victims of this kind of abuse. Rosemary, who was born in 1918, was dyslexic and may have had slight mental retardation. In spite of this, she was able to complete her degree in Montessori education and, at first, live a rather typical life.
However, Rosemary’s father had her lobotomized in 1941 because she was deemed difficult to control and had a short fuse. The treatment was widely hailed as effective for reducing “excessive urges” at the time. The result was tragically devastating for the young lady. She became incontinent, babbled like a toddler, and was largely confined to a wheelchair after the lobotomy, and she spent the remainder of her life in a sanatorium.
Despite mounting skepticism and criticism, lobotomies were nevertheless often performed in the United States up until the late 1960s. At least 40,000 individuals there had the surgery done, many of them against their will and without understanding the full ramifications. For whatever reason, the Soviet Union was the first country to outlaw lobotomy in 1950, citing concerns that the procedure “contradicted the principles of humanity.” Next came Germany, then Japan.
Physics Blunders and “Half-Measures”
The number of Nobel Prizes awarded to physicists proves that the field deserves its reputation as a precise science. Why? Because the winners virtually never got it totally wrong. But there is at least one example of a right approach leading to the incorrect result, and a father and son who are oddly complementary to one another.
The Element Hesperium, Discovered by Fermi

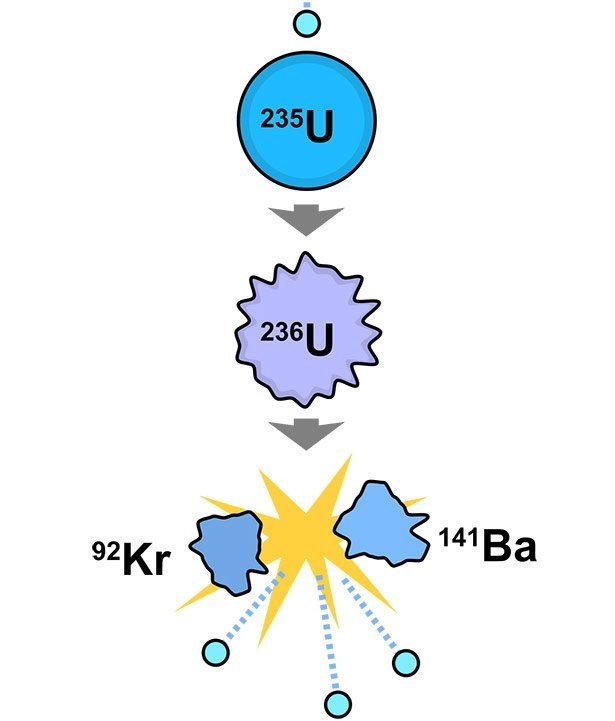
Famous scientist Enrico Fermi is one such example; he helped create the first atomic weapon. In 1938, his work showing that new radioactive elements may be generated by bombarding heavy atoms with neutrons won him the Nobel Prize in Physics.
In theory, that is correct. This is due to the fact that the bombardment results in nuclear fission, which in turn results in lighter decay products, such as those formed from uranium. Fermi, on the other hand, thought he had created brand-new, heavier elements. The new element “hesperium,” with atomic number 94, was discovered by him and his colleagues at the University of Rome in 1934. Hesperium was created by bombarding thorium and uranium with neutrons.
Unfortunately, he was dead wrong; plutonium, element 94, was found a few years after his prediction. However, Fermi’s experiments did not result in the creation of this element. Lighter elements like barium and the noble gas krypton were all that could be extracted from his neutron bombardment. Fermi acknowledged the error during his Nobel acceptance speech, but he was still awarded the prize.
Wave or Particle
Two members of the same family, father and son, Joseph John and George Thomson, won Nobel Prizes in physics. The first subatomic particle, the electron, was discovered by Joseph Thomson, the father, and was awarded the Nobel Prize in 1906. Even as far back as the 1830s, scientists recognized that tiny, charged particles were responsible for transmitting electricity. Thomson’s research with electrically charged gases supported the particle nature of these units.
George Paget Thomson, his son, won the Nobel Prize in 1937 for seemingly doing the reverse, demonstrating that electrons behave like waves rather than particles. He and Clinton Davisson performed an experiment showing how electron beams are diffracted by a crystal lattice, an effect that is common in radiation. Because of wave-particle duality, we now know that electrons may act as both particles and waves.
Meredith Stanley, Tobacco Mosaic Virus

The blunder of a Nobel winner may have even slowed the progress of a pivotal breakthrough. That’s because his alleged proof had everyone convinced for almost a decade that proteins, not RNA or DNA, contained the essential code of life.
What Are Viruses Made Of?
During the 1930s, many biochemists were obsessed with answering the following question: Where is the secret to life hidden? This code would guarantee that offspring would look like their parents and that bacteria would always have similar structures. Meanwhile, doctors were left wondering whether the newly found viruses were indeed alive despite their microscopic size. Maybe they were just molecules after all.
U.S. scientist Wendell Meredith Stanley said in his 1946 Nobel talk that the enigma surrounding viruses was compounded by the fact that their sizes overlapped with those of both biological creatures and chemical molecules. They may have been inorganic, formed of hydrocarbons or carbohydrates, fatty, protein-rich, or even biological in nature; nobody knew.
Infectious Crystals of Protein
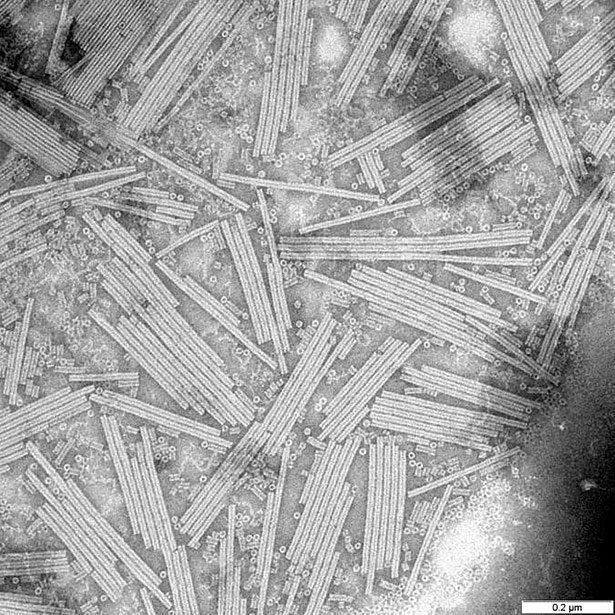
Because of this, Stanley began looking for ways to determine how viruses are put together. In the middle of the 1930s, he was able to crystallize tobacco mosaic viruses (TMVs). The crystals, in Stanley’s opinion, were composed entirely of viral protein. The crystals caused the mosaic disease to spread when a scientist infected tobacco plants with them.
The crystalline substance “contained all the viral activity contained in the infected fluid,” Stanley added. This was a huge step forward since it indicated that viruses are composed of proteins and that the instructions for their reproduction and possible alterations are encoded within those proteins.
The First Steps in Molecular Biology
For his work on the characteristics of the crystalline tobacco mosaic virus, Stanley was awarded the Nobel Prize in Chemistry in 1946. Forty years later, scientist Lily Kay of Johns Hopkins University in Baltimore said, “His discovery has been viewed – with some justification – as the symbolic beginning of molecular biology.”
Stanley had done an excellent job of disproving the conventional wisdom of his day, which held that only live creatures like bacteria could cause disease. His ability to crystallize viruses and precisely describe their characteristics established beyond a reasonable doubt that viruses are not living cells. Therefore, molecular constructs have the potential to spread disease.
However, There Are a Few Small Issues
Kay argues that “nevertheless, Stanley’s work was fraught with technical errors and fallacies.” What Stanley didn’t realize was that his viral crystal samples were anything but pure. They carried the genetic information of the tobacco mosaic virus thanks to the 6% viral RNA they contained. However, Stanley was unaware of this and therefore assumed that proteins were the determining factors in infectivity and viral activity.

Since then, proteins have been the primary focus of those looking for the life code. Canadian doctor Oswald Avery’s 1944 experiment demonstrating that bacteria transfer genetic information through DNA was originally unsuccessful in overturning the accepted wisdom. Not until Alfred Hershey and Martha Chase showed in the early 1950s that bacteriophages replicate inside their host organisms through DNA did this begin to alter.
Thus, it became evident that the nucleic acids DNA and RNA, and not proteins, must be the vehicles for the genetic information of many viruses and all other species. The DNA era officially began with James Watson and Francis Crick’s 1953 descriptions of the molecule’s structure.
Biochemical Confusions
Several Nobel laureates, including Stanley himself, have been proven inaccurate or very slightly off base in their understanding of biological macromolecules. In particular, enzymes were a source of consternation since it was not always obvious whether they facilitated synthesis or encouraged degradation.
The Faulty RNA Enzyme
The biosynthesis of RNA and DNA was recognized with the Nobel Prize in Medicine in 1959, shared by Severo Ochoa and Arthur Kornberg. A few years before, Ochoa had isolated the enzyme polynucleotide phosphorylase from a bacterium. The ability of this enzyme to construct RNA from its constituent parts was shown in vitro.
Based on this reasoning, Ochoa thought it to be pivotal in “transcribing” DNA’s blueprints into mRNA. It was likely that polynucleotide phosphorylase was involved in the production of RNA because of its extensive prevalence in nature, as he stated in his Nobel talk.
In a Cell, They Do Things a Little Bit Differently
What Ochoa didn’t know, and what the Nobel Committee didn’t know at the time, is that the findings from the lab are not directly applicable to what occurs in live cells. Ochoa won the Nobel Prize for work that, under normal conditions, involves the degradation of RNA in cells rather than its synthesis. This is because polynucleotide phosphorylase normally acts in this way.
It was subsequently discovered that the enzyme RNA polymerase is responsible for RNA synthesis in cells. Biochemist Roger Kornberg of the United States was awarded the Nobel Prize in Chemistry in 2006 for his work correcting Ochoa and elucidating RNA production in great detail.
Repairing as Opposed to Building From Scratch

Nobel Laureate Arthur Kornberg’s enzyme theory was somewhat more sound than Ochoa’s. Specifically, he had isolated a type of the enzyme DNA polymerase, which is responsible for making copies of DNA. The fact that this is only successful when a whole DNA strand is available as a model was first noticed by him. Adding the enzyme to the DNA building blocks alone has no effect.
In his Nobel talk, Kornberg said, “The enzyme we are studying is unique in that it accepts instructions from a template.” The fundamental mechanism by which our DNA is replicated was thus uncovered by him. But Kornberg wrongly thought that this process would also generate additional chromosomes in the cell. His discovery of DNA polymerase I helps solely to repair chromosomal DNA damage. DNA polymerase and its variations are essential synthesis enzymes, but they weren’t discovered until his son Thomas Kronberg’s research.
References
- “Johannes Fibiger – Biography”. Nobelprize.org. 30 January 1928.
- Bengt Jansson. “Controversial Psychosurgery Resulted in a Nobel Prize”. Nobelprize.org.
- Jenell Johnson, 2014. American Lobotomy: A Rhetorical History. University of Michigan Press.
- Lawrence K. Altman, 2007. “Arthur Kornberg, Biochemist, Dies at 89”. The New York Times.