For the first time ever, a laser fusion experiment has successfully ignited nuclear fusion, creating more fusion energy than was required to heat the fusion fuel. The experiment at the National Ignition Facility in the United States has reached a new record high in terms of heating energy, marking the first time it has ever exceeded the fusion breakeven threshold. Even though there is still a good distance to go before fusion power plants can generate energy, this is a significant step forward for fusion research throughout the world.
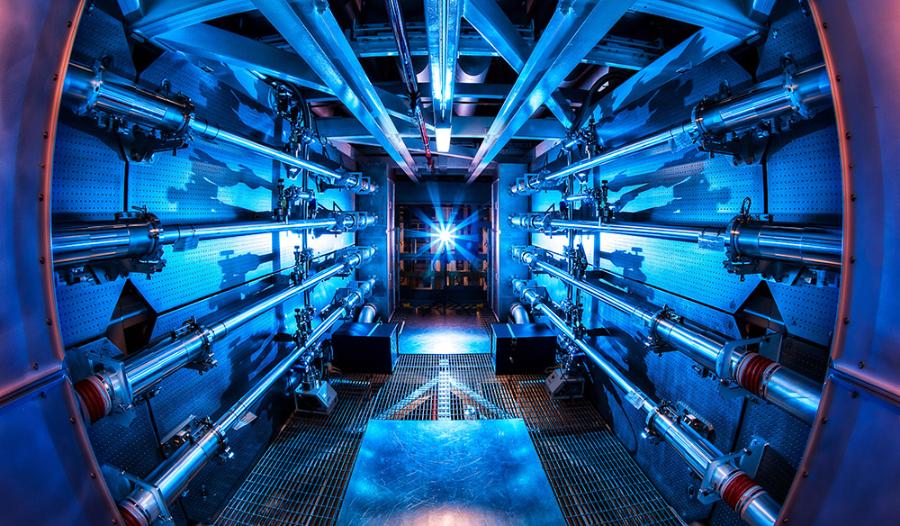
Since nuclear fusion is expected to become a major energy source, substantial resources are being devoted to its study. JET or the soon-to-be-built, large-scale reactor ITER are all test reactors that use magnetically contained and heated plasma for this purpose. The National Ignition Facility (NIF) at the United States’ Lawrence Livermore National Laboratory uses microscopic hydrogen or deuterium-tritium pellets that are crushed by laser bombardment to bring about fusion.
Finally, laser fusion produces more energy than is required to heat it.
To date, however, not a single fusion technology has been able to achieve the so-called “break-even” threshold Q. This is the point at which the energy output by the plasma in a fusion reactor equals the energy input. In order to overcome this barrier, fusion must be ignited, which is a critical first step on the path to producing energy via nuclear fusion.
Laser fusion achieves a crucial milestone
Researchers at the National Ignition Facility have finally succeeded in doing so. A new record of 3.15 megajoules of fusion energy was created in an experiment. Intense laser pulses were used to heat the deuterium and tritium nuclei to a temperature where the stored energy could be released, and this process only needed “just” 2.
05 megajoules of heating energy.
The energy output from a fusion reaction exceeded the input.
It’s the first time the fusion plasma in NIF’s small fuel capsule has ignited and, in terms of heating energy, hit the breakeven threshold. It is one of humanity’s biggest scientific challenges to date to ignite nuclear fusion. The accomplishment is a credit to science, engineering, and, most significantly, the individuals who worked for it. This goal has motivated scientists for the last 60 years.
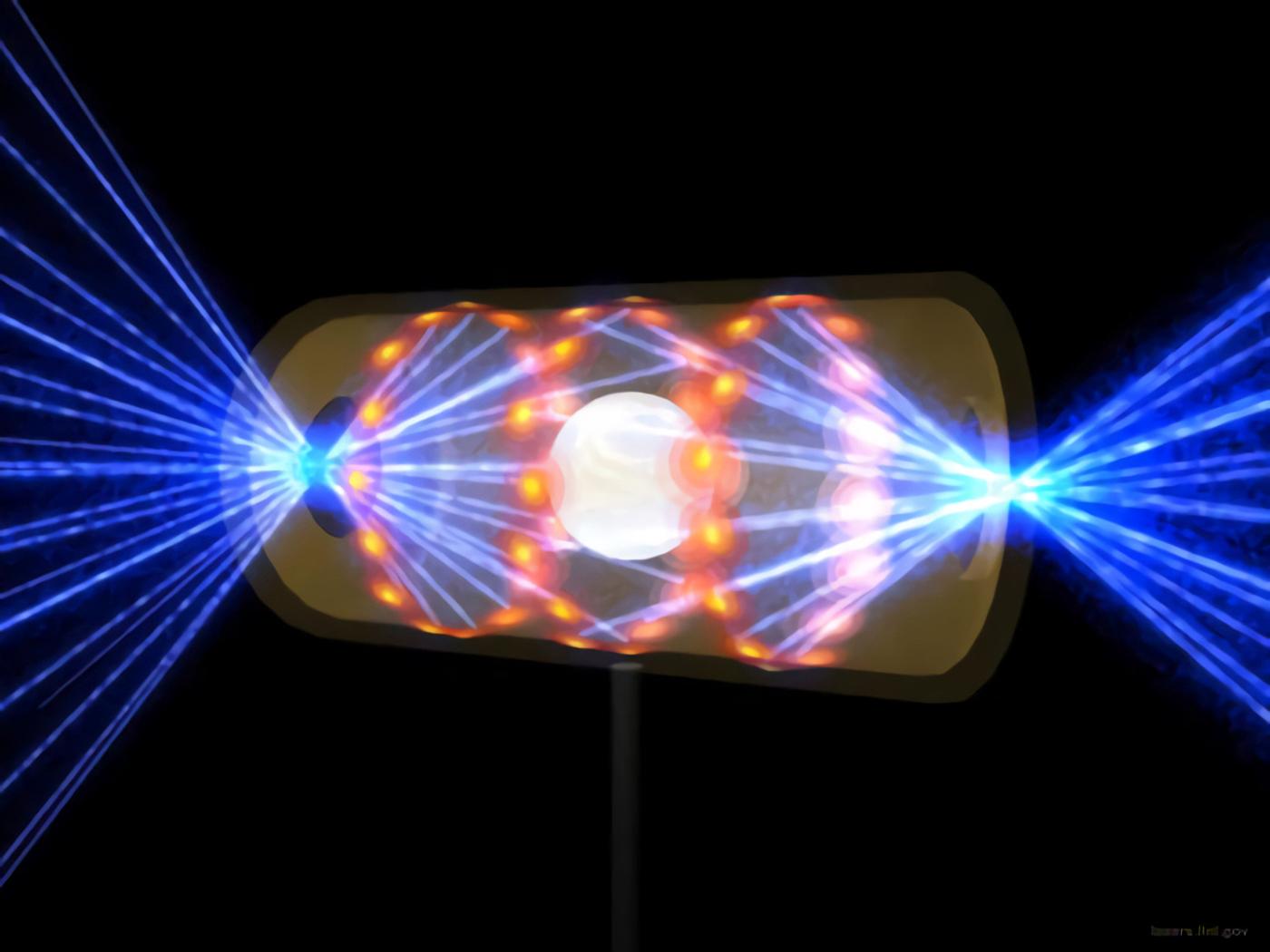
Mechanisms of the laser fusion device
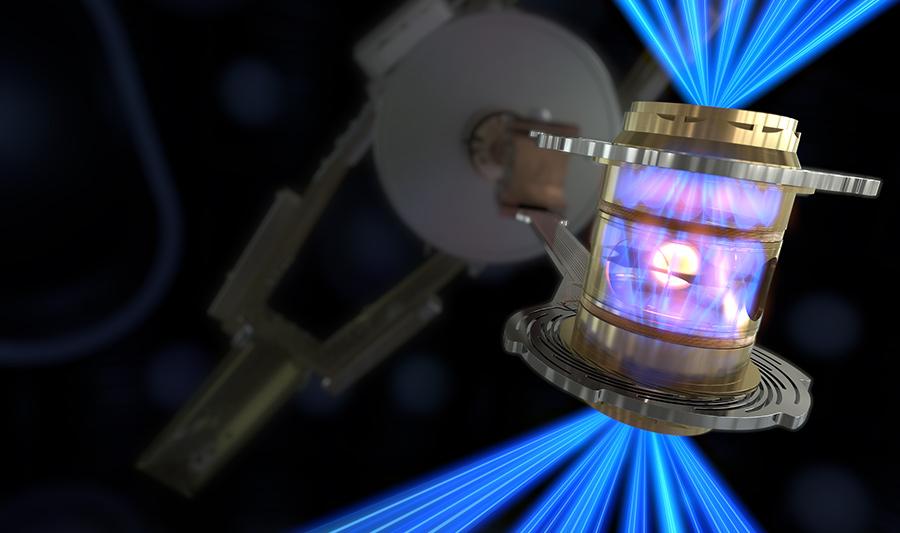
The 192 high-powered neodymium lasers at the fusion plant at the National Ignition Facility are the primary source of energy. Their infrared light source is transformed into UV laser pulses with a narrowly focused 351-nanometer spectrum by a series of amplifiers and lenses. The fuel capsule in the middle of the reactor chamber, which is just a few millimeters in size, is struck with up to 500 trillion watts of energy from the pulsed and focused laser beams.
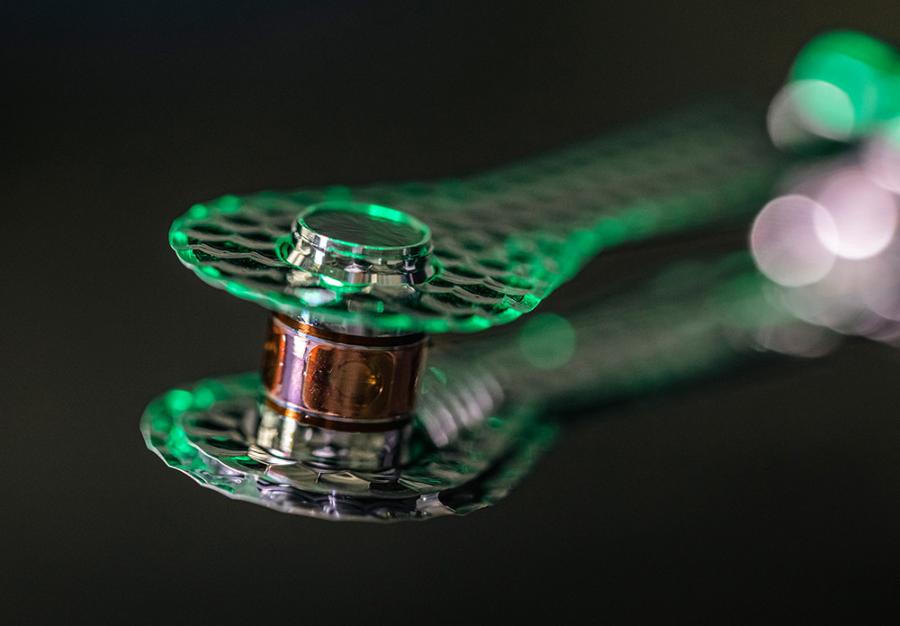
Within a tiny chamber is a capsule made up of the hydrogen isotopes deuterium and tritium; this is the fusion fuel. The fuel capsule is spared from the focused laser pulses, which instead hit the cavity’s inner walls. In that location, high-energy X-rays are produced and aimed at the fuel capsule from all angles. Therefore, the capsule material warms up to roughly 120 million degrees in a matter of billionths of a second and expands inward.
The sudden increase in pressure causes the deuterium and tritium fuels to fuse. In order to achieve ignition, fusion energy must reach 1.3 megajoules, which this facility did in August 2021. After this point, fusion can continue to occur without any further energy being added. Since then, the physicists have successfully modified the cavity’s original form in order to focus more X-rays on the fuel capsule.
Crucial steps forward in fusion research
Today, fusion was successfully ignited. A self-sustaining fusion reaction occurred for a brief period of time (picoseconds) until the plasma thinned out again due to expansion and fusion ceased. Reaching the breakeven threshold is a major accomplishment for physicists at the National Ignition Facility, and it puts laser fusion at the top of this research area once again. Simply put, this breakeven threshold has not yet been reached by any other experiment or test reactor.
Nuclear physicists point out that this finding is still far from the energy increase that would be required to create electricity. But 500 megajoules of electrical energy was used to power the lasers in order to provide 2.
05 megajoules of heating energy to the target. And the 3.15 megajoules of fusion energy is still far less than what was previously required.
In addition, the laser system used in this achievement had to be equipped with over 7,500 specialized lenses, each measuring several meters in length, in order to produce and direct laser energy towards the intended target. In the past, the NIF system has only been activated once per day. To be really effective, though, a laser fusion power plant would need to ignite as many as twenty times every second.