In December 1952, the capital of the United Kingdom, London, experienced an unprecedented air pollution event known as the “Great Smog of London.” Lasting for five days, this smog severely impacted London’s transportation, daily life, and health, leading to thousands of deaths. It stands as one of the most severe air pollution events of the 20th century, serving as a pivotal moment in triggering environmental awareness and legal reforms.
Background and Causes of the Great Smog
The backdrop of the Great Smog of London was the industrialization and urbanization process in the United Kingdom. Since the late 19th century’s industrial revolution, London became a thriving center of industry and commerce, with a continuous increase in population and buildings. Coal, serving as the primary energy and fuel source, was extensively used for power generation, transportation, manufacturing, and heating.
The immediate cause of the London smog was the unusual weather conditions in November and early December of 1952. London experienced rare cold and windless weather, with temperatures below freezing, high humidity, and overcast skies. This weather led to an atmospheric phenomenon known as “temperature inversion,” where the air temperature at higher altitudes was higher than that at lower altitudes, preventing vertical convection and creating an “inversion cap” that trapped London’s air and pollutants near the ground.
In this situation, London residents, in their pursuit of warmth, burned a significant amount of low-quality coal containing high levels of sulfur and ash. This coal combustion produced more particulate matter and sulfur dioxide. These pollutants were unable to disperse and instead reacted with water vapor and other chemicals in the air, forming toxic substances like sulfuric acid, hydrogen chloride, and fluorides. These substances adhered to the particulate matter and condensed into droplets, creating dense smog.
—>The atmospheric pollutants, such as sulfur dioxide and sulfur dioxide, emitted from sunlight, thermal power plants, diesel vehicles, etc., lingered in the cold air, concentrating and forming a highly acidic sulfuric acid fog with a pH of 2. The maximum concentration of sulfur dioxide was around 0.1 ppm during normal times, reaching 0.7 ppm during the smog event, and the concentration of suspended particulate matter exceeded 1.7 mg/m3 from the usual 0.2 mg/m3. Source
Impacts and Consequences of the Great Smog

The effects of the Great Smog of London were devastating. Starting on December 5th, London was covered in a thick yellowish smog, reducing visibility to just a few meters or even centimeters, accompanied by the smell of rotten eggs, making breathing difficult both outdoors and indoors.
The smog posed significant challenges to London’s transportation and daily life. Public transport, including the subway, buses, taxis, and trains, suffered severe disruptions, with many routes forced to shut down or experience delays. People had to resort to walking or cycling, but even these activities were difficult because of poor visibility.
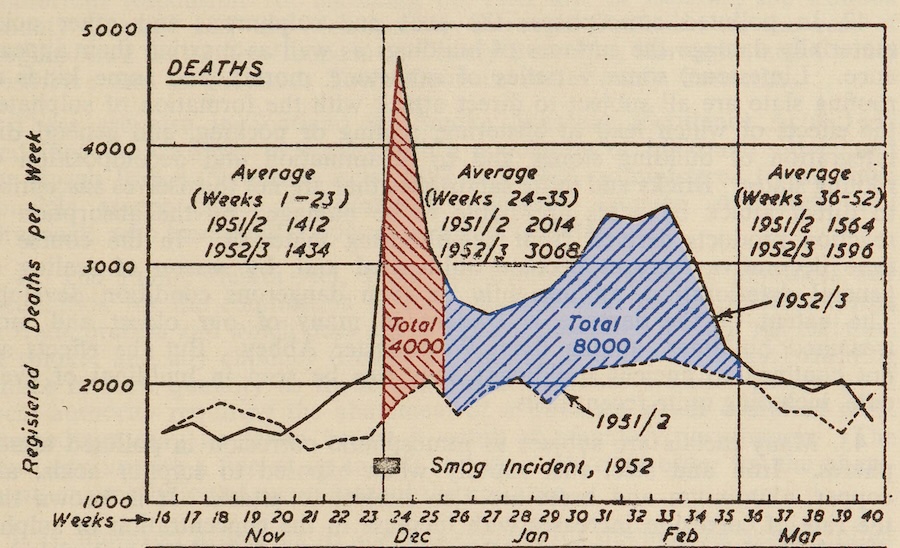
The health consequences of the smog were severe. The toxic substances in the smog irritated people’s eyes, throat, nose, and lungs, causing respiratory diseases such as bronchitis, pneumonia, asthma, and emphysema. Statistics indicate that during the smog event, London’s mortality rate doubled compared to normal times, with an estimated 4,000 deaths occurring that month and subsequent death toll estimates potentially reaching as high as 12,000. Source
Reflection and Reforms Following the Great Smog
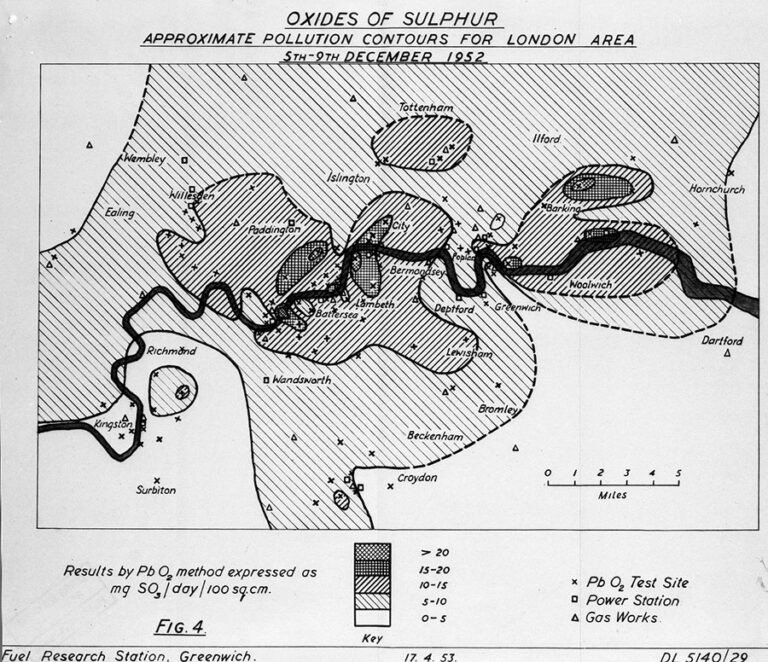
The Great Smog of London prompted heightened awareness and reflection on air pollution and environmental protection. People recognized the costs of industrialization and urbanization, as well as the drawbacks of coal as an energy and fuel source. Efforts were initiated to seek cleaner, more sustainable development methods and more effective air quality monitoring and management systems.
—>When the fog lifted, an assessment was conducted, revealing that during the days of extreme smog, the death toll in London had nearly tripled. The death rates for individuals aged between 55 and 65 increased by 142 percent, while those in the 65 to 75 age group rose by 235 percent. The maximum SO2 concentration reached 3.82 milligrams per cubic meter of air.
The smog event led the UK government and parliament to enact a series of laws and measures aimed at reducing air pollution and preventing similar events. Most notably, the Clean Air Act of 1956 was passed, marking the UK’s first law specifically addressing air pollution and one of the earliest environmental laws globally. The act established standards and limitations for coal burning, encouraged the use of smokeless coal or alternative energy sources, created smoke control areas, imposed fines on violators, provided subsidies to affected residents, regulated emissions from factories and power plants, and implemented air quality monitoring and reporting.
—>One significant action was the drastic reduction in the number of open fireplaces. However, the implementation was slow, leading to another, less hazardous smog in 1962. Subsequently, additional measures were adopted from 1968 onwards (Clean Air Act 1993).
Internationally, the Great Smog of London drew attention and responses from the global community, fostering the environmental protection movement and cooperation worldwide. Many countries and regions also formulated similar laws and policies to control and reduce air pollution, improve air quality, and safeguard human health and life.
Researchers Clarify the Cause of the London Killer Fog

—>There were work stoppages, revenue losses in public transportation and among taxi drivers. Additional costs incurred due to the necessary street lighting during the day and the deployment of 18,000 extra police officers sent to London, as there was a significant increase in thefts and robberies.
Now, researchers claim to have discovered what made the weather so deadly: the combination of specific weather conditions with exhaust particles that turned into poison. Diminishing winds were the beginning. During days of calm winds in early December, chimneys from power plants and factories blew warm air upward, while winter air cooled the ground.
Soon, a fatal layer of mild air formed, sitting above the cold air like foam on beer. The two air layers mixed little, creating inversion weather. At ground level, pollutants accumulated, hanging over the city like a dirty bell. Less and less sunlight reached the ground, which continued to cool until the cold air couldn’t hold moisture anymore. The moisture gathered on particles, condensing into fog.
The air at ground level continued to cool, prompting Londoners to heat up more. Sulfur emissions from coal combustion filled the haze from chimneys and factory stacks; this was already known. However, researchers wanted to understand why sulfur dioxide turned into toxic acid. Their concern was that the deadly fog could recur in smog-plagued Asian cities.
The crucial ingredient, they found, was nitrogen dioxide, as reported by scientists led by Gehui Wang of the Chinese Academy of Sciences in the Proceedings of the National Academy of Sciences. The substance also forms during coal combustion and causes sulfur dioxide to turn into sulfuric acid when combined with water.
In the London fog, another coincidence triggered the chemical process, the scholars wrote: the air was exceptionally humid due to rare weather conditions, allowing a lot of acid to form. When the water droplets later evaporated, they left behind acid in a toxic concentration that people eventually inhaled.
In China, however—that’s the good news—the smog air is not acidic, the study’s authors note. Apparently, at least one ingredient of the complex London toxic recipe is missing.
A crucial difference is likely that the fine particles on which the fog in Asian metropolises settles are smaller; consequently, less acid forms in the tiny water droplets.
But even in China, large amounts of the fatal ingredient nitrogen dioxide enter the air; hence, the researchers warn: The right interplay of all factors can create deadly fog elsewhere, not just in London.
Featured Image: Illustration, Malevus.com