High-pressure studies show that the Earth’s deep core may include oxygen in the form of an unusual, iron-rich oxide in addition to iron and nickel. Two layers of iron atoms and one layer of oxygen atoms alternate in an iron oxide, which might develop at the high pressure and high temperature of the Earth’s core. To explain why the Earth’s core is softer than solid metal, this hitherto undiscovered chemical may be responsible.
Aside from outer space, the inner, solid core of the Earth is the most intense and mysterious spot in our world. This is due to the fact that very little is known about the composition of this solid metal sphere beyond the fact that it contains iron and nickel. However, there have been growing signs over the last few years that the Earth’s deep core is softer than previously assumed and must therefore also include lighter elements like hydrogen, sulfur, silicon, carbon, oxygen, and maybe even the noble gases helium and xenon.
Complications with oxygen
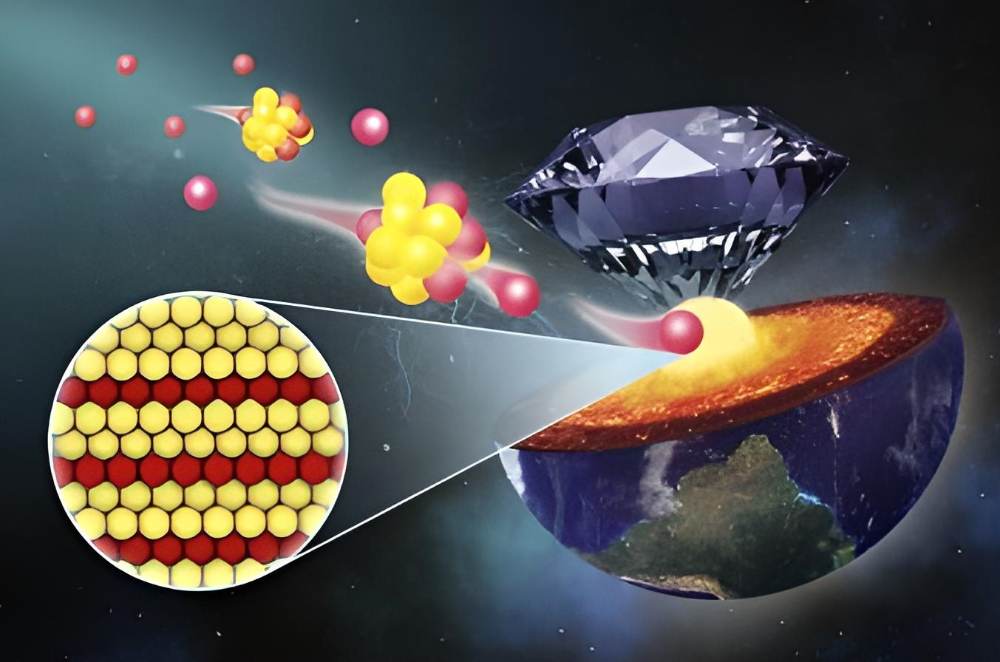
Light elements, such as oxygen, are thought to exist somewhere towards the core of the Earth. Chemical interactions in the deep interior include oxygen transmission between the core and mantle, as explained by the researcher Jin Liu of Beijing, HPSTAR. Oxygen was present at every stage of Earth’s creation and development. In spite of the fact that iron and oxygen are widely acknowledged as crucial ingredients in planet formation, the existence of oxygen in Earth’s deep core remains disputed.
Some scientists hypothesize that unique high-pressure iron oxides contain oxygen and might be found in the Earth’s deep core.
The issue is that until recently, only low-iron varieties of iron oxide – the most probable form of oxygen in Earth’s core – were known. However, they do not correspond to the iron-rich interior of Earth. Conversely, it is generally accepted that pure oxygen is insoluble in solid iron. In order to simulate the high pressure and temperature found in the Earth’s core, the scientists conducted high-pressure tests to see whether iron-rich iron oxides could be created under such conditions.
Researchers used a diamond anvil cell to subject iron powder and iron oxides (FeO or Fe2O3) to pressures of up to 260 gigapascals and temperatures of 4,900 to 5,800°F (2,700 to 3,200°C).
Iron oxide with novel properties at the core of the Earth
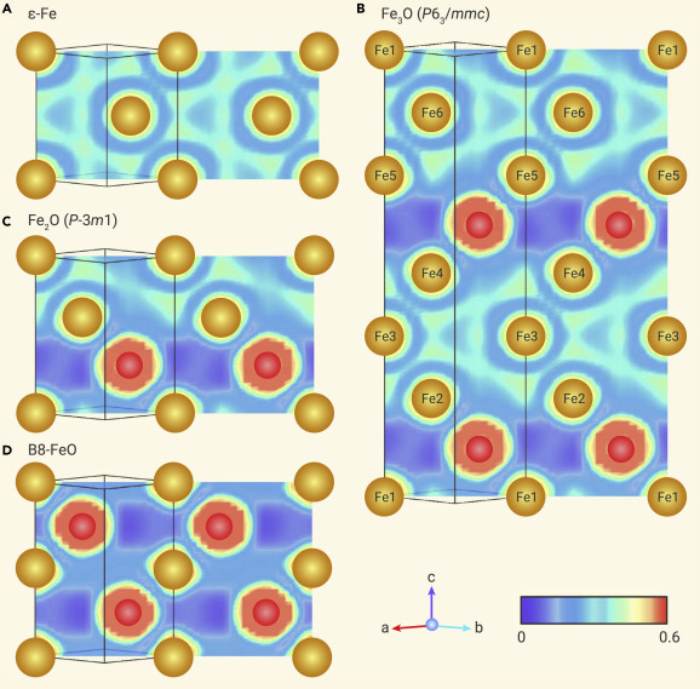
Since nothing significant was occurring in the sample chamber during the first runs, even at temperatures of 4,170°F (2,300°C) and pressures of 220 gigapascals, the results were not encouraging. A chemical reaction did not seem to have occurred. When the material, however, reached the very high pressures and temperatures at the center of the Earth, things changed. Researchers claim that X-ray scattering analysis revealed peaks that did not correspond to any previously identified iron oxides or iron carbides.
Analyses showed that in the high-pressure chamber, Fe28O14, an iron-rich iron oxide, had been produced. Unlike other iron oxides, the oxygen and iron atoms in this compound’s crystal structure are not randomly scattered around. Instead, they stack in discrete layers, one of oxygen atoms after two of iron atoms. The atomic structure as a whole is a tight hexagon.
Having properties more akin to metal than oxide
Based on these findings, the team concludes that iron-rich iron oxides may be stable in the extreme conditions of the Earth’s deep core. Therefore, oxygen may be present in Earth’s inner core and may explain the seismic “softness” of the solid core as one of the light element admixtures. This would also explain why preliminary investigations show that the iron in these iron-rich iron oxides seems to behave more like a metal than a conventional oxide.
Further research into the anisotropic iron oxide compounds’ elastic, rheological, and thermal transport capabilities might shed light on the seismic aspects of Earth’s deep core, its creation and composition, and the thermal development of our planet. High-pressure iron oxides are thought to exist in both the outer and inner cores of the Earth, although little is known about their precise characteristics or the nature and extent of their presence.