When Antoine Lavoisier or Antoine-Laurent de Lavoisier started his chemistry studies in the 1760s, Aristotle’s four-element theory (earth, water, air, and fire) still dominated the discipline. At the time of Lavoisier’s death thirty years later, this discipline had been transformed into a science that was practiced in modern chemistry experiments.
Who Was Antoine Lavoisier?
Antoine Lavoisier was the son of a wealthy attorney and received formal training at the College des Quatre-Nations in the city, usually known as the College Mazarin. He studied mathematics under the supervision of astronomer Abbe Nicolas Louis de Lacaille after finishing his humanities education in 1760. On another occasion, he would explain his mathematician-like attention to detail and care when conducting scientific studies. “They never prove a proposition before the previous step becomes clear. “Everything is linked, everything is connected, from the definition of the point to the definition of the line and the supreme realities of transcendental geometry.“
He left the college in 1761 and studied law to please his father, but his interest in science continued. He studied meteorology with Lacaille and continued to work in this field even after his teacher died in 1762. After observing the northern lights (Aurora borealis), he published his first scientific paper in 1763. He attended chemistry courses given by pharmacist Charles Louis La Planche at the Pharmacists Association, public classes on electricity by Guillaume-François Rouelle, and Bernard de Jussieu’s lectures on the plant kingdom.
He studied mineralogy, geology, and chemistry with Jean-Etienne Guettard, a member of the French Academy of Sciences. He received a law degree in 1764 and was accepted as a lawyer. But he never worked as a lawyer. In fact, he was already planning for the transition to the Republic of Science.
The following year, in 1765, he presented his first paper on “the analysis of gypsum” to the Academy of Sciences, where he went as a guest scientist. He showed how solid gypsum turns into powder when heated and creates steam during the process. He gathered the steam and found that it was pure water. This was crystallization water, with a quarter of its solid weight being heated. When he mixed the water with powdered gypsum, it turned into a solid mass again.
So, the source of the stiffness of gypsum was water. By weighing and measuring ingredients and products during synthesis and analysis, Lavoisier proposed the “double demonstration” method that he would use throughout his science career. His paper was published in the proceedings of the Academy. About a year later, he published a second paper on gypsum, showing that it consists of chalk and sulfuric acid and that its solubility depends on acid concentration. Lavoisier insisted that the analysis of mineral substances is important to shed light on Earth’s past.
Academician and Tax Collector
In 1776, the Academy announced a public competition for the best method to illuminate the city streets. The participants were required to present a theory adapted to calculations, physical and chemical experiments, and practice. Lavoisier studied all kinds of lighting meticulously, looked at lamps of different sizes and types, and tested them with oil and wax. In his report, he concluded that olive oil was the best fuel. The paper was awarded a gold medal. Antoine Lavoisier said with great energy that he wanted to put his energy, intelligence, and knowledge to work for the state.
In 1767, with a long field trip to the Vosges Mountains with Guettard, he collected data for his extraordinary work, “The Mineralogical Atlas of France.” For more than four months, they roamed the land, analyzing the soil, minerals, and water and taking samples from agricultural products. There were 26 maps, eight of which belonged to Lavoisier. He was elected to the academy in 1768.
Lavoisier joined the private company Ferme Generale (General Farm) by purchasing shares, which also collected tax revenues on behalf of the King of France. The company was collecting tax on goods entering Paris, such as salt, tobacco, and alcohol, as well as the customs money, with a six-year revenue rental agreement with the king’s Controller-General of Finances. They were paying a certain sum to the royal treasury in cash, which was close to 150 million libres in 1770.
Lavoisier was particularly responsible for the Tobacco Commission, where he was tasked with preventing fugitives and cheating at retailers. Then he had much more important tasks to complete. Jacques Paulze de Chastenolles was a strong and wealthy manager. In 1771, her 14-year-old daughter, Marie-Anne Pierrette Paulze, married the 28-year-old Lavoisier. Maria brought lots of dowries with her and became Lavoisier’s assistant. She learned chemistry and took notes while Lavoisier conducted experiments. She also translated English chemistry books for him and hosted many scientists who came to visit Lavoisier. They had no children.
Antoine Lavoisier and the Four Elements

Lavoisier installed a well-equipped laboratory in his first house in Paris, the Rue des Bons-Enfants. The distinctive feature of his work was the quantitative approach based on the constant use of chemical balance. The law of conservation of mass has long been the basic principle. He would later say in his textbook: “The whole art of experimenting in chemistry rests on this principle; in all experiments, one is obliged to assume an actual equality between the principles (that is, elements) of substances examined and those obtained by the analysis of these substances.“
He applied the axiom for the first time in his paper “Nature of Water,” dated 1770. At that time, many chemists believed that water could be turned into soil. Because it was known that when the distilled water was evaporated in the glass container, there was always a soil residue. Lavoisier weighed the glass container called a “pelican” and poured in water that he had distilled eight times, then measured its weight.
He kept the container at 70 degrees Reaumur (80 degrees Reaumur is the boiling temperature of water). After 101 days, he began to identify dust particles in the container, but there was no change in the temperature of the water. Also, the weight of the container had decreased, and the weight loss was equivalent to the weight of the powder particles. It was clear that the powder on the surface of the glass container was dissolved in water. Moreover, Antoine Lavoisier found that water did not turn into soil.
Lavoisier has moved on from Aristotle’s Four Elements and is now experimenting with air and fire. He decided to find out what happened to the air during combustion. By using air and water, he would also examine a second process called calcination. When metals are heated, a lime-like substance forms on the surface of the metal; chemists thought this type of calcination was kind of slow-burning.
Both calcination and burning were thought to be caused by fire, or more specifically by its main component, phlogiston—the enigmatic substance named by German chemist Georg Ernst Stahl. Nobody had detected phlogiston, but it was thought to be present in everything that was burning, at different rates. Oil and coal were said to be almost pure phlogiston.
When substances burned, phlogiston was released from the substance, producing fire. The same process was thought to produce calx during the calcination. But Lavoisier wasn’t too happy with this statement as the final weight of the burned metal was more than the weight of the initial metal when its weight and calx’s weight were added together.
If phlogiston was thrown out of the metal, then how could the burned metal be heavier? Antoine Lavoisier suspected that something was added to the metal to form calx and it could be the air.
The Birth of Modern Chemistry
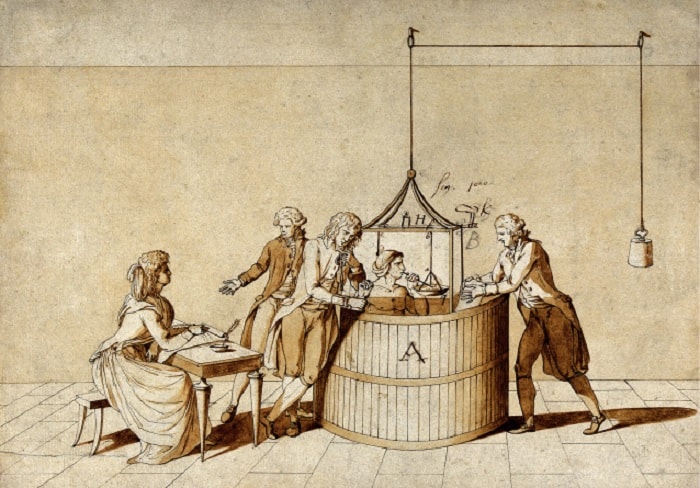
Antoine Lavoisier delivered a sealed envelope to the Academy of Sciences on November 1, 1772, to be opened in 1773. He found that when sulfur and phosphorus were burned with air, their weight would never decrease, in fact, it would actually increase. Moreover, when the lead calx, called litharge, was treated with charcoal, it lost weight and released a large amount of air.
Early in the next year, he wrote the memorandum of a long series of experiments that he intended to begin to clarify the role of gases in chemical combustion. One of his aims was to discover whether the air involved in combustion and calcination was atmospheric air or a special kind of air, then known as “fixed air,” that was named by Scottish chemist Joseph Black (we know it as carbon dioxide today). The answer to this question would revolutionize chemistry.
British scientist Joseph Priestley assisted Lavoisier in starting this revolution. In 1774, he went to Lavoisier’s house in Paris for dinner and told him that he had discovered a new kind of air. While working on the mercury calx, he had found that air was extracted from the calx as it turned back into metal, and this air had completely different properties than Black’s “fixed air.” Priestley called it “air without phlogiston.” But Lavoisier did not resort to phlogiston, as he understood the meaning of this phenomenon better than Priestley. Unlike other calxes, this new gas could not come from charcoal, as mercury calx did not require charcoal to return to metal; it should have come from the calx itself.
On April 26, 1775, Lavoisier proudly reported to the Academy of Sciences that “the principle [substance] that combines with metals during their calcination is the purest part of the air.” That “fixed air” results from the combination of the highly respirable part of the air with charcoal. He later called this “purest part” “oxygen,” which means “acid producer” in Greek, because he (wrongly) thought that all acids contain oxygen.
In 1783, British scientist Henry Cavendish burned hydrogen in a closed container to obtain water. He accurately concluded that water was created from the burning. But since he still believed that oxygen does not contain phlogiston, he thought the missing substance was supplied by hydrogen. In the presence of many members of the Academy, Lavoisier repeated Cavendish’s experiment and showed that hydrogen and oxygen form water when burned together.
He argued that water is a combination of two gases, not one element. In 1785, he carried out a major experiment on the analysis and synthesis of water; the experiment both validated his discovery and enabled the development of hydrogen as a large-scale production method. (Soon after, Lavoisier would realize how hydrogen was a better lifting gas while refereeing a hot air balloon competition between the Montgolfier brothers and another inventor.)
There was nothing left of Aristotle’s theory of the four elements of earth, water, fire, and air, and there was no such thing as phlogiston. In 1787, Lavoisier, Claude Berthollet, Antoine Francois de Fourcroy, and Louis Bernard Guyton de Morveau published the new Chemical Nomenclature, which would change the way we think about chemistry. Two years later, in his book Traite elementaire de chimie, Lavoisier summed up his ideas and gave a list of the known elements.
The Chemistry of Life: Oxygen

Antoine Lavoisier was now exploring the physiology of the human respiratory tract. He concluded that breathing was kind of slow-burning. Therefore, the oxygen in the air must be essential for the chemistry of life. Lavoisier and Pierre Simon-Laplace invented the calorimeter to measure the heat emitted from an animal.
He compared this to the heat released during coal combustion and calculated the animal’s energy consumption. He then compared animal and human oxygen consumption while inactive and also on the move. Lavoisier created two papers on the physiology of animal respiration.
Lavoisier was one of the first to predict the value of the chemical approach to the physiology of nutrition and the mechanisms of tissue anabolism. In the 1790s, he was ready to start a second scientific revolution in biology. He sensed the vital role of the liver in synthesis. In fact, he had created a conceptual study program, and it would take almost 100 years for science to develop it.
Why Was Antoine Lavoisier Executed?
The French Revolution began in 1789, and Lavoisier was drawn into the process of demolishing the General Farm, which was thought to represent the worst excesses of the regime and was hated by revolutionaries. All his contributions in other areas had been forgotten, including:
- Significant progress was made in gunpowder production under his management.
- Agricultural reform began thanks to his model farm in France, the first scientific farm.
- Efforts to enforce the metric system.
- Participation in the Art and Trade Advisory Board.
- Deep thoughts on public education.
- Efforts to save France from bankruptcy.
- His collection of memories, Regional Welfare of the French Kingdom, is a touchstone in the history of economics.
During the worst days of the Reign of Terror, on May 28, 1794, the Jacobins, thinking that all officers of the tax farm were against the revolution, caught 28 government representatives of the national treasury and tried them in the Revolutionary Tribunal.
All were found guilty and executed by guillotine at the Place de Revolution. Antoine Lavoisier was the fourth. The great mathematician Joseph Lagrange commented on this event: “It took only a moment to cause this head to fall, and a hundred years will not suffice to produce its like.“
Bibliography:
- “Lavoisier, Antoine Laurent”. Lexico UK English Dictionary. Oxford University Press.
- “Lavoisier”. Collins English Dictionary. HarperCollins. Retrieved 30 July 2019.
- (in French) Lavoisier, le parcours d’un scientifique révolutionnaire CNRS (Centre National de la Recherche Scientifique)
- Schwinger, Julian (1986). Einstein’s Legacy. New York: Scientific American Library. p. 93. ISBN 978-0-7167-5011-6.









