They all want blood, whether it’s Dracula, Lestat, or Nick Knight. An eternal mystery, the legend of blood has long captivated the human race. Numerous civilizations have unique myths and stories about the crimson elixir of immortality. “Blood is a very unique fluid,” Mephistopheles said. Therefore, he insisted Faust use a “drop of blood” to seal the pact. But what makes blood so special?
Science, following in the footsteps of religion and literature, has endeavored to unravel the mystery of the life force that courses through our veins.
Death is an inevitability when blood loss is excessive. The question then becomes why blood is so important and what role it plays in the human body. Our blood volume is between 5 and 6 liters. So where does the blood flow originate from?
Transferring oxygen from the lungs to the body’s numerous tissues is the blood’s primary role. The carbon dioxide is transported back to the lungs on its return journey. Blood does play a role in gas transportation, but it is not the only mechanism. It serves as a highway for many other compounds to reach their destinations in the body. For instance, hormones fall under this category, but so do essential nutrients for metabolic function.
Toxic waste products are carried away from the body by the blood to the liver and kidneys, where they are eventually eliminated. And it’s vital to the body’s ability to fight against invaders.
Blood of myth
As it relates to rituals, religions, and supernatural
There is a common thread connecting myths and religions across the globe, and that thread is the story of blood. Homer likely has the first account of a blood-related magical ritual. On his way to meeting the spirits of the dead, Odysseus must first sacrifice a number of animals. The only way the deceased can remember the hero’s name and face again is if they consume this elixir.
Vampire mythology certainly isn’t exclusive to the West. The Germanic peoples, the Romans, the Celts, and even the Africans all had ideas about the undead slurping off human blood.
They were called “Lamias” by the ancient Greeks. Women who died in mysterious circumstances were said to be resurrected as vampire-like ghosts. They devoured the victims and drank their blood.
However, blood has always played an important symbolic role in both literature and religion. Both the ancient Chinese and the Egyptians had the belief that consuming the blood of a murdered enemy would bestow upon the drinker the same supernatural abilities as the deceased. Legend has it that Germanic peoples used the still-warm blood of slaughtered bears, wolves, and cattle as a currency to purchase these animals’ territories.
Killing for the sake of the blood
Some religions practiced blood sacrifices in which human or animal lives were sacrificed as an act of worship. Blood was worshiped as a deity’s beverage among the Mayans. Because of this, it was necessary for there to be a constant flow so that communication with the gods and ancestors would be maintained.
Ancient Egyptians and Babylonians shared this view, thinking of blood as the conduit between the body and the afterlife. Even in the Old Testament, blood is connected with life. During the Inquisition, lamb’s blood was painted on walls to ward off bad spirits and as a reminder of the atoning power of animal sacrifices. However, at the Lord’s Supper, we partake in the symbolic drinking of Christ’s blood for the purpose of receiving forgiveness for our sins.
For a long time, blood was also a common way to symbolize intimate relationships. For instance, the term “blood brotherhood” refers to the unique closeness shared by two buddies that normally exists only between biological brothers.
Blood, along with phlegm, black bile, and yellow bile, was thought to be one of the four lifebloods back in the Middle Ages. They believed that disturbances in the equilibrium of these four humors were at the root of all diseases. Excess blood has to be drained to restore equilibrium. However, until the nineteenth century, physicians still routinely performed bloodletting as one of the most prevalent therapies for any and all ailments, even after the four-fluid hypothesis had become obsolete.
The discovery of blood circulation
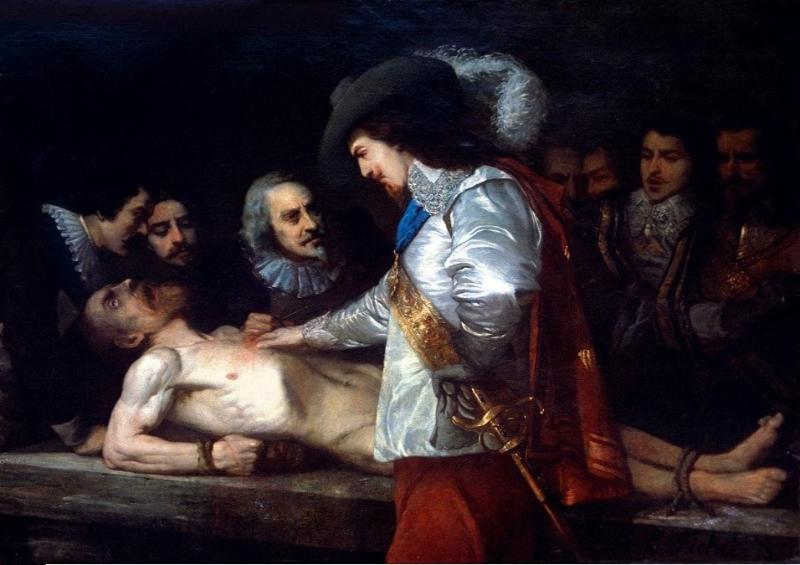
For a long time, little was understood about blood, despite its central role in literature and religion. What exactly is blood composed of? What causes its structure to take shape? These mysteries were solved years later by diligent scientists. Up until that time, the common belief was that food was used to make blood, in accordance with Galen’s teachings.
British physician William Harvey made the discovery of blood flow in the first part of the 17th century. The blood needs a propelling device, such as a pump, to prevent it from pooling in the legs due to gravity. The heart is responsible for carrying out this process. Blood can only flow one way through the heart, and the heart valves make sure it’s out of the body and away from the heart in the arteries and back in via the veins.
But how can we explain the constant circulation of blood? Instead, the blood flow should slow to a near stop between contractions before being pushed forward once again. Blood arteries are no longer hard tubes, thanks to nature’s solution of making them stretchy. So, the veins close to the heart get more blood between heartbeats and then release it all during expansion. The blood is essentially discharged in a wave form from the heart before being transformed into a steady flow.
The individual cells found in the blood
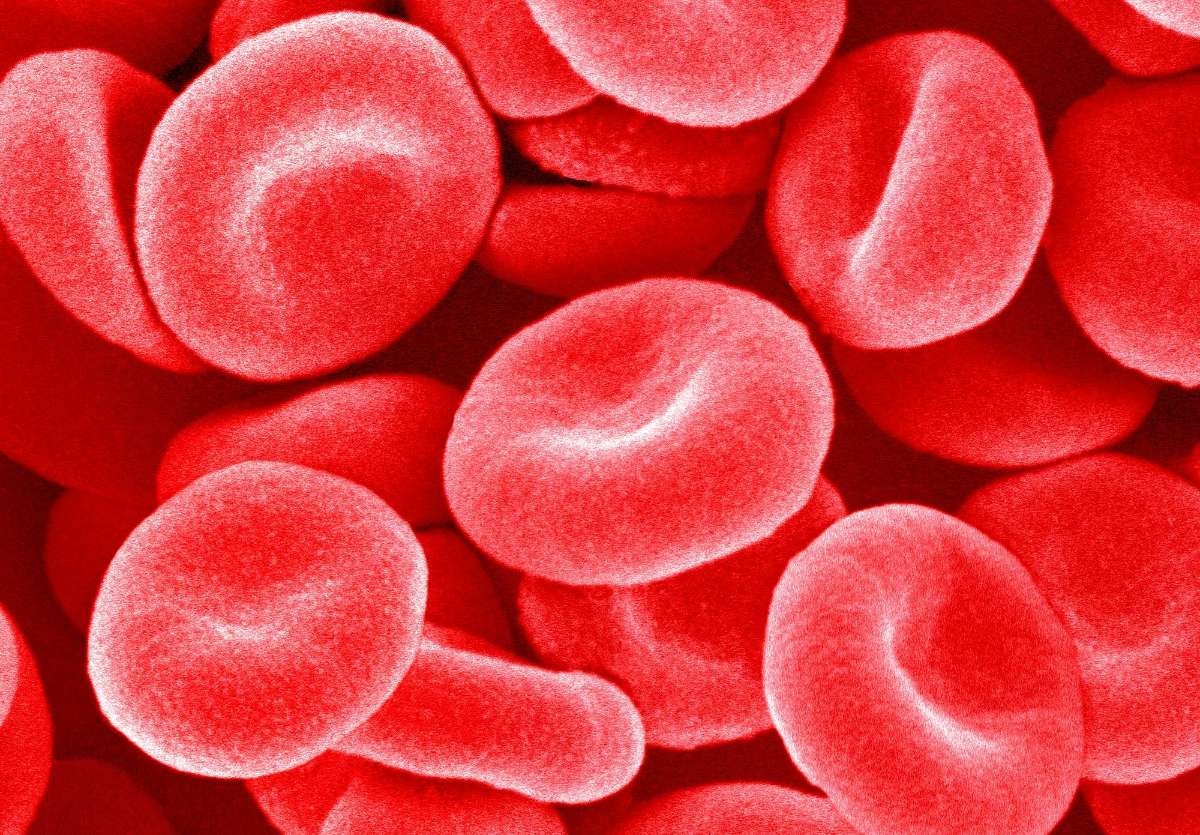
Blood is made up of separate cells. If you scratch your finger and look at the drop of blood under a microscope, you will discover that blood is not a homogenous liquid. A few are white, while the rest are bright red. Because blood is both a fluid and an organ, every component has a unique purpose. It’s not only a conduit for oxygen and nutrition, but also for waste products like carbon dioxide and chemicals.
This is where your body’s first line of defense stands against invading cells and organisms. The “juice of life,” then, contains a cast of characters. White blood cells, such as B cells, T cells, and natural killer cells, act as “soldiers” and “guard troops,” mingling with the “pure transport workers” like hemoglobin, which carries oxygen.
Red blood cells (erythrocytes)
Erythrocytes are the primary component. The form of a red blood cell is similar to an egg’s, albeit it is somewhat flatter in the center. Hemoglobin, an iron-containing protein that gives blood its red hue, is responsible for carrying oxygen to the body’s tissues. During this step, the heme groups’ iron molecules bind with the oxygen molecules.
Reduced hemoglobin levels lead to the condition known as anemia. There are a number of possible reasons for this anemia, but in the Western world, a deficiency of iron in the blood is the most common. Iron deficiency is more common in women because of menstruation, although iron-poor diets (such as those followed by vegetarians) may also lead to anemia.
White blood cells (leukocytes)
The blood contains both red and white blood cells. Disease prevention relies on the immune system, which includes white blood cells. They are not only present in the blood, but also in the lymph. Antibodies are produced by B cells, which subsequently bind to any foreign substances, such as viruses or bacteria, that have entered the body.
Upon detection, so-called T-helper cells signal for the elimination of these binding complexes. When a virus is the offending agent, T-helper cells dispatch killer cells to do away with the contaminated tissue.
Platelets (thrombocytes)
Despite popular belief, there are more components to blood than just red and white blood cells. If a vein or artery were slashed, blood would spurt out in all directions, and the victim would bleed to death. Platelets’ function is to stop this from happening. They start blood coagulation by closing up damaged blood arteries.
Maintaining a consistent concentration in the blood is essential for their functions. Bleeding may occur if there are not enough thrombocytes in the blood, and thrombosis can develop if there are too many. It’s the formation of clots in the blood vessels that prevents the normal circulation of blood.
Blood plasma
Blood cells and thrombocytes still need a liquid medium to move across the body. Plasma in the blood does this work. It consists of almost entirely water and has a 96% water content. Immunoglobulins, which transform into antibodies after an immunological response, are part of the four percent of plasma proteins.
Blood coagulation
Healing of bare cuts by themselves
Humans have the remarkable ability to swiftly create a scab over any cut or scratch. This scab is made entirely of dried, coagulated blood.
It stops bleeding from broken blood vessels. In the normal course of events, platelets produce a hemostatic plug a few seconds after damage, which is then strengthened by a tiny network of fibrin fibers. Fibrin acts as a hemostatic and initiates blood vessel healing. The blood does not naturally contain it, but rather it is generated via a series of precursors involving over 30 distinct elements before it can be found there.
Liver dysfunctions are particularly dangerous for blood clotting since the liver is responsible for producing several of the clotting components. In reaction to damage, clotting is initiated by signals sent only locally. The ultimate goal is to keep the blood from clotting entirely, since this would render it useless as a transport medium.
However, there is no guarantee that bleeding will cease after an injury. If you look at leeches, for instance, they can draw five times their body weight in blood in half an hour without the bite wound clotting. Their method is to secrete hirudin into the wound while they feed, which keeps the blood supply so the leech can keep sucking. Hirudin binds to and neutralizes thrombin in the circulation, preventing it from initiating a clot.
Patients are already being given hirudin and related medicines to prevent thrombosis after surgery or during long-distance flights.
Nonetheless, chemical compounds aren’t the only things that may stop blood from clotting. Some individuals are born without this defense system functioning properly. They have hemophilia, a disorder that runs in their family. When an injury develops, no scab will form, and the wound will continue to bleed unchecked, perhaps causing a lot of blood loss. Most notably, members of European royal families have been affected by the inherited bleeding disorder hemophilia.
Hemophilia
In late 19th-century England, Queen Victoria married her daughters into the most powerful governing houses on the continent in an effort to solidify her position there. Soon, babies were born. However, many of them, particularly male kids, had to overcome a severe disability: Whenever they were hurt, their blood poured non-stop. On top of that, the wounds just weren’t healing. Among them, only a few grandchildren of Queen Victoria reached adulthood. So many of them died either soon after birth or even before they turned 18.
So how did this mysterious illness that mostly targeted males rapidly spread among Europe’s royalty (kings, dukes, etc.) over such a huge geographical area? The sick, power-mad queen was actually the source and the original cause of this disease.
The mutation of an X-chromosomal gene encoding a blood clotting factor is identified as the root cause of so-called hemophilia. It is generally accepted that males have just one X chromosome and females have two X chromosomes. Due to its recessive inheritance pattern, the condition does not manifest itself if the second X chromosome’s intact gene can generate a functional clotting factor.
Because of this, women who have the abnormality on just one X chromosome are not afflicted with the illness but are carriers. However, there are no clotting factor genes on the Y chromosome in men. Therefore, if the X chromosome gene is faulty, it cannot be substituted by a normal gene on another chromosome.
The son of a healthy mother who is a carrier has a 50% chance of inheriting the faulty chromosome and the sickness from her. This is because males obtain their X chromosomes from their mothers. To develop the condition, a female must inherit a faulty chromosome from both her parents. If this is the case, however, it means that the father is a hemophiliac as well.
Hemophilia was uncommon in women since male hemophiliacs seldom achieved sexual maturity, particularly in the past. The number of women who are infected with HIV has grown, despite the availability of improved treatment alternatives. Even though a cure has not been found, the illness may be managed with frequent injections of the clotting factors.
The blood group system
Medical professionals always insist on finding out a patient’s blood type before administering a transfusion, no matter how dire the situation is. Just how crucial is it that the patient obtain blood from someone with the same blood type as them? Because of the high mortality rate prior to the identification of blood groups, blood transfusions were only sometimes carried out. Due to this, they were outlawed in several European nations around the turn of the last century.
However, Viennese physician Karl Landsteiner (1868–1943) wondered if there was a pattern to the transfusions’ inconsistent performance. He combined his own blood with the blood of five coworkers in a wide variety of ways. His work on this led to the development of the blood group system in 1901, and he was eventually given the Nobel Prize for his efforts.
Blood groups A, B, and 0 serum include “clumping proteins,” which bind to red blood cells of other blood types and cement them together, causing a blockage in the blood arteries. The antigens on the surface of red blood cells are the proteins that are there. Erythrocytes that have a distinct surface make up are identified as invaders and attacked. The key-lock mechanism makes this possible. Specific antibodies are produced by lymphocytes, which then bind to foreign antigens to create an insoluble complex.
Rhesus factor
Landsteiner’s research greatly assisted in the care of injured servicemen during World War I, among other areas of medicine. In spite of this, he sometimes encountered obstacles beyond explanation, compelling him to delve further into the crimson elixir of life’s mysteries. It wasn’t until 1940, when working with rhesus monkeys, that he realized there was still another factor in compatibility. This rhesus factor was discovered by isolating a previously unknown component of the red blood cell membrane.
Having or not having (Rh+) is the underlying premise (Rh-). To what end, however, does the rhesus factor serve as a proxy for blood safety? Hemolysis may occur in a patient with an Rh-negative blood type if a donor with a positive Rh factor is used. This results in the complete elimination of RBCs (red blood cells) from the blood.
When a patient receives donor cells, their own immune system rejects them because the patient’s cells lack the rhesus factor. That is, they generate anti-rhesus factor antibodies. A group of immune cells gets a message from the antigen-antibody complex that an invading pathogen must be dealt with. Specialized T-killer cells eliminate the complex by destroying it. Hemoglobin is removed when the cell membrane has been destroyed. The opposite is not true, though. Therefore, Rh+ individuals are safe to receive blood transfusions from Rh- donors.
Potential dangers to the unborn
Furthermore, the Rh factor is crucial during pregnancy. Generally, things go well with the firstborn. A woman who is Rh- but has given birth to a kid who is Rh+ will have antibodies in her blood that attack the Rh+ cells. If she conceives another kid who is Rh+, the antibodies will cross the placenta and cause hemolysis in the baby.
Rh incompatibility used to be a leading cause of stillbirths. To prevent the immune system from developing defense cells against the child’s blood, doctors now vaccinate mothers with anti-Rh antibodies right after the birth of the first child. This vaccination is given again in the second and third trimesters of pregnancy.
Twenty distinct blood types have been discovered in humans. The AB0 and Rhesus systems are two of the most popular.
Risks of blood transfusion
The curse of blood
Unfortunately, blood carries more than just hormones, oxygen, and nutrients. Once within the body, pathogens may travel through the bloodstream to other organs with equal efficiency.
Although blood transfusions save lives, they pose a danger if the blood provided is not adequately screened for infectious agents and compatibility. This has been recognized at the very least since the HIV-contaminated blood crisis of the 1980s. Standard testing for HIV and hepatitis viruses, for instance, did not become accessible until the late 1990s.
Hepatitis
In the late 1970s, after giving birth, around 7,000 women received an anti-D immunoglobulin vaccination in one country to protect against rhesus factor incompatibility in future pregnancies. However, roughly 2,500 women got hepatitis C because the virus was present in tainted batches.
Meanwhile, hepatitis infection during transfusions is less likely due to systematic testing of blood supplies. Infections through tainted blood units happened often before dependable diagnostics for hepatitis viruses were available. Because it might take ten to twenty years for normal symptoms like fever and jaundice to manifest, many blood donors are unaware that they are carriers of hepatitis viruses.
Then, if the illness does spread, there are many important stages it might take. In most people with hepatitis B, the illness clears up on its own when the body develops antibodies to combat the virus. However, chronic development occurs in 5 to 11% of instances. Patients also generate some anti-hepatitis B antibodies, but not nearly enough to drive the virus out of liver cells.
Hepatitis C poses a much greater risk since, unlike hepatitis B, there is currently no vaccination available to prevent the disease. Liver cirrhosis occurs in severe instances. After successfully cultivating the hepatitis C virus in cell culture, scientists still have high hopes of discovering new treatment options.
West Nile Virus
According to legend, ravens began dropping dead from the skies just before Alexander the Great’s death, a sure sign that he had contracted the West Nile virus. In 1999, a similar event happened in New York City’s Central Park. The virus had flown over the Atlantic to the West, where it quickly disseminated throughout the Americas. The virus has also been a recurrent topic of discussion in the European media.
The West Nile Virus was first discovered in 1937 in Uganda’s West Nile District, hence the name. Birds are the primary hosts, and they may be found in both tropical and temperate regions. However, mammalian species, including humans, are rapidly becoming its victims. More than 4,000 cases were recorded in the United States in 2002, with 284 deaths. Blood transfusions were the source of infection in 13 instances.
The Culex pipiens mosquito is the primary transmitter of the West Nile virus. Scientists pondered the mystery of the virus’s rapid spread across the Atlantic. More research into the mosquito species led scientists to discover that two subspecies call Europe home. One likes to bite people, while the other likes to bite birds. However, an intermediate variety that bites both birds and people in the Americas makes for an efficient viral carrier.
At first, it was thought that the virus could not spread from person to person. But since the beginning of the millennium, evidence has accumulated to support the idea that it is likely spread by the blood during transfusions and organ transplants.
Avoiding unnecessary risks requires a few weeks of the waiting period after returning from North America between June and November before a donor may give blood again. Epidemics tend to happen in the summer because the disease and its carriers both multiply more quickly when temperatures are higher.
West Nile encephalitis brain tissue courtesy of the Centers for Disease Control and Prevention
The majority of infected people show no symptoms, but those in the 20 percent who do have flu-like symptoms. Danger arises when the virus causes meningitis by crossing the blood-brain barrier. No effective cures still exist at this time.
Fighting malaria with sickle cell anemia
Life-saving genes that are also deadly
There are moments when nature seems to follow its own peculiar set of rules. That’s what the researchers who identified the relationship between malaria and sickle cell anemia must have believed. The latter is an inherited disorder brought on by a change in the hemoglobin gene. This causes the red blood cells to take on an abnormal sickle form, which causes them to get lodged in the arteries and prevent oxygen from reaching the affected organs. A heart attack happens when blood arteries get clogged and no longer provide blood to the heart.
Darwin’s “Survival of the Fittest” theory predicts that harmful mutations should be rare and eventually eliminated by natural selection. The prevalence of sickle cell anemia in malaria-endemic areas like West Africa is a curious situation. Scientists discovered that the altered cells provide a selective advantage in some environments. The malaria parasites only target RBCs. However, sickle cells are more susceptible to destruction, leading to the simultaneous death of both the infections and the host cells.
Deadly mosquitoes spread disease
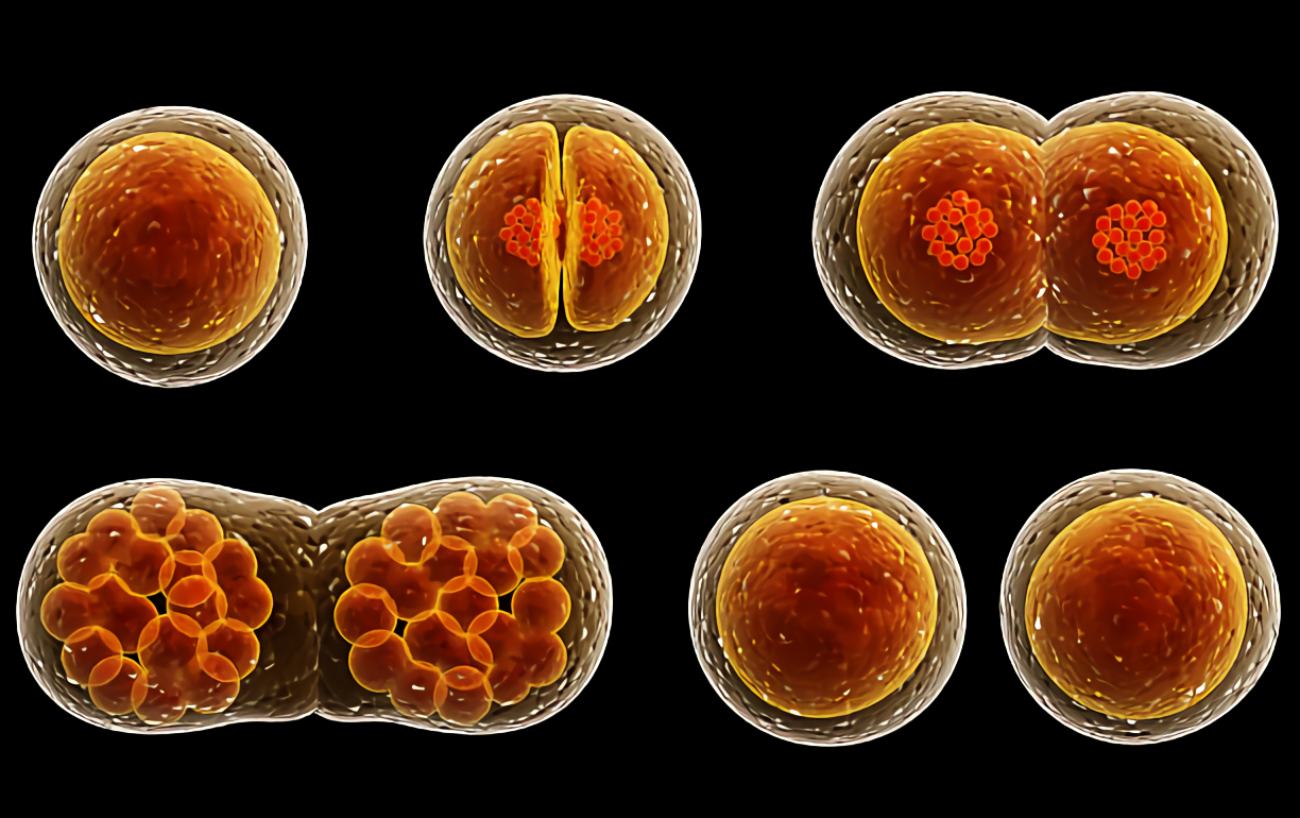
The mosquito-borne illness malaria is well-known across the world. The Anopheles mosquito is responsible for spreading the parasitic protozoan Plasmodium. When a mosquito bites an infected person, it takes in the plasmodia along with the blood. Transmission from one victim to the next takes place by physical touch.
Once inside a host, parasites will go toward the liver to multiply. Once within the red blood cells, the daughter cells grow rapidly before being ejected in a violent burst. As a consequence, malaria is associated with recurrent fevers, for which it is recognized.
There are two phases to the malaria parasite’s life cycle: the mosquito-borne sexual cycle and the human-borne asexual cycle. Female and male plasmodia fuse in the Anopheles mosquito. The so-called sporozoites form in the egg between eight and sixteen days after fertilization and then enter the bloodstream of a human host during the subsequent sucking cycle.
They develop into schizonts in the liver cells, the subsequent intermediate stage. Plasmodium falciparum (Malaria tropica) is the most severe form of the disease, in which all schizonts grow into cells whose daughter cells damage liver cells and then infect erythrocytes in the blood.
Only a subset of schizonts reaches this stage of development in the mild and harmless malaria forms. The remainder goes into a hibernation-like state in the liver, where it may remain viable for a long time. In time, they develop into adults and cause the condition to recur.
The parasite is very well-protected since it is able to evade immune system assaults by hiding out within the host’s liver and blood cells at all times. Furthermore, it generates a protein whose composition intermittently changes, making immune system adaptation difficult.
Expectations for a vaccine
The Plasmodium and Anopheles genomes were first sequenced by a group of scientists in 2002. Antimalarial medications exist, however, malaria parasites rapidly evolve resistance to these treatments.
As of 2022, the RTS,S, also known under the trade name Mosquirix, is the only malaria vaccine that has been licensed for use anywhere but the European Union. Developed in 1987, RTS,S received WHO approval for “wide usage” in children only in 2021.
Attacks on the immune system
The immune system is to contemporary people what the heel was to Achilles. It keeps harmful bacteria at bay, but when it breaks down, disaster strikes. Specifically, the white blood cells are the “wonder spot” because they are the first line of defense against infections. The consequences of their destruction or damage are catastrophic. Disabling the immune system leaves the body vulnerable to pathogens it would normally be able to fight off.
AIDS
This is the root of the AIDS epidemic, which has been likened to the Black Death in Europe in the 14th century. They are working around the clock to stop the spread of the illness, which is affecting Africa particularly badly.
The HIV virus is responsible for the disease; it is a kind of retrovirus whose genetic material is encoded in RNA but becomes fixed as DNA inside the host cell’s genome. When a virus integrates its genetic information into a host’s own, it becomes very tough to eradicate. It can only reproduce in the presence of CD4-positive cells throughout the body.
It is the T-helper cells that are principally responsible for cellular immunological defense in humans. Following the integration of viral DNA into the helper cell’s nucleus, the cell is coerced into accepting new instructions. The T helper cell is no longer producing immune response-coordinating messenger molecules, but rather HIV viruses, which eventually cause the cell to die. As a result, the released HIV viruses infect and hijack more T helper cells.
During this period, the body’s immune system is constantly fighting off infections. About ten billion new viruses are created each day by infected T-helper cells, and one billion T-helper cells each day die and need to be replaced. At first, this works well, but eventually, your body just can’t make enough T-helper cells to replace the ones you lose every day. The patient is now open to infection from any germ since their immune system is no longer functioning.
After around 10 years, the decreased immune system might cause a variety of illnesses. It is generally accepted that AIDS sets in when the CD4 cell count drops below 200 per microliter. What this implies is that the body is open to contracting deadly infections. Thanks to modern medical knowledge, we may put off this process and save ourselves the trouble it might bring. Unfortunately, there is currently no hope for a cure.
Hope for a cure
Vaccine development for HIV is still the primary focus of ongoing studies. Protein molecule Gp120 is the entry point for HIV viruses into infected immune cells. Gp120 changes its structure in response to binding the CD4 receptor. Knowing how gp120 changes shape leads scientists to suppress HIV by employing medicines that impede the shape shift. But as of 2022, HIV is still not curable but only controllable with HIV treatment.
HIV may be transmitted by any kind of skin-to-skin contact that results in a blood transfusion. Blood supply contamination has been a major concern for quite some time. However, in the meantime, testing blood donors for HIV has become routine. It takes many weeks after infection for antibodies to build in the blood, so there is still a certain residual danger. After that, the HIV test is able to detect them.
White blood cells
When compared to the decline of lymphocytes seen in AIDS, the other extreme is seen in leukemia, when an unchecked proliferation of one or more kinds of white blood cells occurs in the bone marrow, but these cells are non-functional. The progenitors of mature lymphocytes are targeted by acute lymphocytic leukemia, whereas granulocytes are the primary target of myeloid leukemia. DNA mutations in the stem cells are the cause of the deterioration.
The mutated cells circulate around the body and eventually end up in different organs. In addition, the cluster of leukemia cells in the bone marrow pushes out the stem cells that normally give rise to all the other types of blood cells. This causes abnormal hematopoiesis, which leads to a shortage of blood cells over time. Weakness, pallor, an increased vulnerability to infection, and a propensity to bleed are all common signs of leukemia.
Current treatments for leukemia include chemotherapy and stem cell transplants. Chemotherapy is the use of strong medications to halt the development and division of cancer cells. These cytostatic medications are effective against cancerous cells, but they can harm healthy organs. Subsequently, patients often have awful adverse effects such as vomiting, mucosal irritation, and alopecia.
A frantic hunt for potential treatments
Because of the inherent dangers of bone marrow or stem cell transplantation, new therapeutic approaches are desperately required. Scientific efforts are presently concentrated on pinpointing optimal targets.
The enzyme DOT1L is an example of a gene activator that plays a crucial role in leukemia development and hence is a possible target for novel medicines. DOT1L limits the activity of the DOT1L enzyme, paving the way for killing the afflicted cells of leukemia patients.
The killer cells of the immune system are the topic of study for experts. Scientists are able to grow in the lab killer cells capable of killing leukemia cells thanks to genetic changes. Motherwort has a compound that kills leukemia cells as well. In this method, normal cells are protected, unlike with present pharmaceutical options. Leukemia is often treated with chemotherapy. The leukemia cells are chemically destroyed by the drugs.
The cause of leukemia remains a mystery after years of study. According to the most widely accepted theory, leukemia develops when certain types of blood cells acquire mutations in their DNA.
Those who have been exposed to ionizing radiation, whether from the sun, X-rays, or radioactive sources, are at a higher risk. However, cytostatics, benzene, other chemicals, and immune system abnormalities may all play a role in bringing on the condition. A hereditary component cannot be ruled out.
Artificial blood
The safe alternative
Donating blood can save lives. Summer is particularly prone to bottlenecks caused by a lack of blood supplies. More blood is required, on the one hand, since accidents are more common among travelers. On the other hand, donors tend to stop donating while they’re away.
However, there are several factors at play, such as the aging of the population, that contribute to the overall decrease in blood donors. Surgical treatments, such as hip replacement, are becoming more common among the elderly as a result of the expanding capabilities of modern medicine. More blood must be stored for this reason.
However, the average lifespan of a blood unit is about 40–42 days. Donated blood is becoming less effective because of the rising prevalence of blood-borne viruses like HIV and hepatitis. Many individuals have been wary of giving or receiving human blood since it was discovered that blood transfusions might spread illnesses like AIDS and hepatitis.
Seeking a substitute that fits
Because of this, doctors are looking for alternatives all the time. The next best thing would be synthetic blood. After decades of research, however, experts conceded that it would be too hard to create a fluid with all the characteristics of human blood. Therefore, scientists are focusing more on two crucial activities, oxygen transportation, and blood loss compensation.
Scientists have come to believe that synthetic blood should be thicker than real blood in order to limit the danger of capillary breakdown. In order to counter this, scientists in the United States have created a format called MP4. Specifically, this makes use of RBCs (red blood cells) extracted from human donors’ blood that have been frozen.
Polyethylene glycol is added to the blood to make the hemoglobin molecules bigger, making the blood thicker. Moreover, MP4 has a stronger affinity for oxygen than other products, so the oxygen is released only in the capillaries, where it is absorbed directly by the surrounding tissue. In theory, artificial blood doesn’t care about your blood type.
On paper, artificial blood is more efficient in its primary function—oxygen transportation—than “real” blood. However, since artificial blood loses 50% of its oxygen-transport ability after a few days, it can currently only be employed for acute applications. But in contrast to donated blood, artificial blood can be kept for a year or longer. Natural blood, on the other hand, has a shelf life of just one month before it deteriorates.
How about hemoglobin extracted from animals?
To anybody who has ever been on the mudflats, the little mounds of sand left behind by the lugworm are instantly recognizable. But who knew that these unassuming creatures would provide a fresh ray of hope in the fight against disease? The worms’ hemoglobin might one day replace human hemoglobin or be used to preserve organs.
The protein hemoglobin in human blood can store four molecules of oxygen. Lungworms’ hemoglobin protein holds 156 oxygen molecules. The lugworm is also a universal donor since its blood lacks the A, B, and O antigens that distinguish different types of human blood.
Pigs’ red blood cells also have been used to successfully create artificial blood. This type of artificial blood has very large molecules for carrying oxygen with characteristics that are similar to those of human blood.
Moreover, when scientists need to boost the blood volume due to blood loss, they add in plasma expanders. This is an ideal substitute for blood. As scientists believe, nothing currently stands in the way of the industrial manufacture of artificial blood.