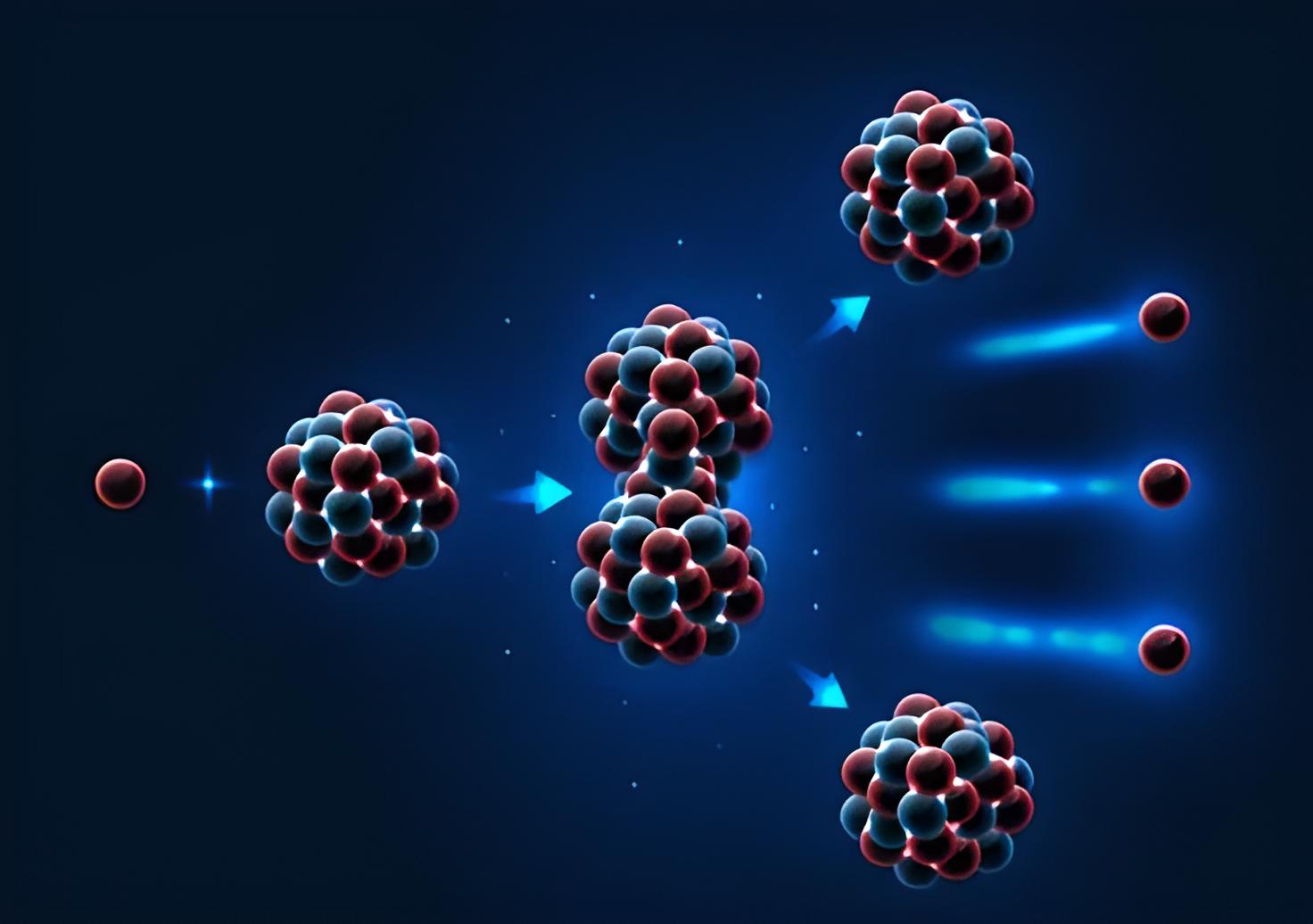
Chain reaction at a glance:
- By bombarding U-235 with neutrons, fission is triggered, creating numerous neutrons and opening the door to a chain reaction.
- However, in order for the fast neutrons created during nuclear fission to once again be likely to divide enough uranium nuclei, they must be slowed down by a moderator (such as water).
- One needs a critical mass of fission material for the chain reaction to continue.
- Reducing the quantity of free neutrons is one way to manage a chain reaction.
The heavy uranium-235 nuclei were initially found to be split by bombardment with slow neutrons (mean speed approx. 1.25 mi/s [2 km/s]) by Otto Hahn (1879–1968), Fritz Strassman (1902–1980), and Lise Meitner (1878–1968). As the uranium-235 nucleus sucks in the slow neutron, an unstable intermediate nucleus (uranium-236) is momentarily created before “bursting” into two fairly heavy nuclear pieces.
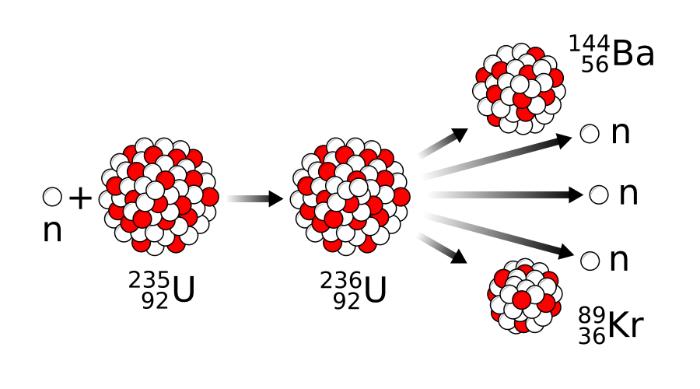
There are two or three extremely fast neutrons (with a typical speed of 6,200 mi/s [10,000 km/s]) produced by this fission process, which, after slowing down, may set off other nuclear fissions (a chain reaction). After fission, radioactive fragments are left behind, which degrade over time.
The nuclear fragments retain some of the binding energy that formerly kept the uranium nucleus together but is now surplus to requirements. Also, due to their positive charges, the pieces are repelled from one another electrically and are propelled apart. A crystal lattice prevents them from readily escaping and instead acts as a powerful brake on their speedy escape. Kinetic energy is transformed into potential energy when a vehicle is slowed down.
About the critical mass
Some of the neutrons emitted must cause nuclear fission, which in turn causes a chain reaction. “Critical mass” refers to the minimum amount of fissile material needed to maintain a chain reaction. Too many neutrons escape at the surface if the mass is below the critical mass, preventing fission from occurring. As a result, the critical mass also relies on the shape of the fissile material. For a sphere, the critical mass of U-235 is about 110 lbs (50 kg). However, the critical mass would be compromised if this quantity of U-235 was laid out flat, rendering a chain reaction impossible.
Slowing down of fast neutrons
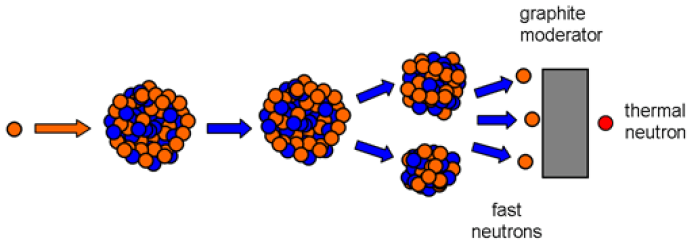
The velocity of a neutron is a key factor in determining whether or not it can break apart a U-235 nucleus. Thermal neutrons are the only kind of neutron with a high enough chance of splitting additional U-235 nuclei to start or sustain a chain reaction. While only fast neutrons are created during U-235 fission, these rapid fission neutrons must be slowed down before they can be used. Moderation describes this procedure.
The numerous hydrogen nuclei (protons) have about the same mass as the neutrons, making water well suited for slowing down the rapid neutrons. Neutrons lose the most energy in collisions with companions of about the same mass (cf. the Newton pendulum). So, in water, fast neutrons slow down to thermal neutrons. Meanwhile, a fast neutron would be reflected by the heavier uranium nucleus.
Moderation of a chain reaction
In order for nuclear fission to be used for peaceful purposes, the chain reaction must be contained and prevented from proceeding explosively. This requires maintaining a steady rate of nuclear fissions per unit of time after the reactor’s “start-up” procedure. This is accomplished by controlling the availability of free neutrons capable of nuclear fission of uranium. There are a number of approaches to this:
- One option is to include boric acid in the water supply used for cooling. The neutron-absorbing characteristic of boric acid is remarkable. The long-term neutron gain is lowered by using this method.
- There’s space between the fuel components for the control rods to go in. In addition, the control rods are constructed from a neutron-absorbing substance (e.g., cadmium). This technique allows for very precise control of a reactor during operation. However, if the rods are entirely placed between the fuel components, the neutron count can be reduced very quickly.
Moderation of fast neutrons by water
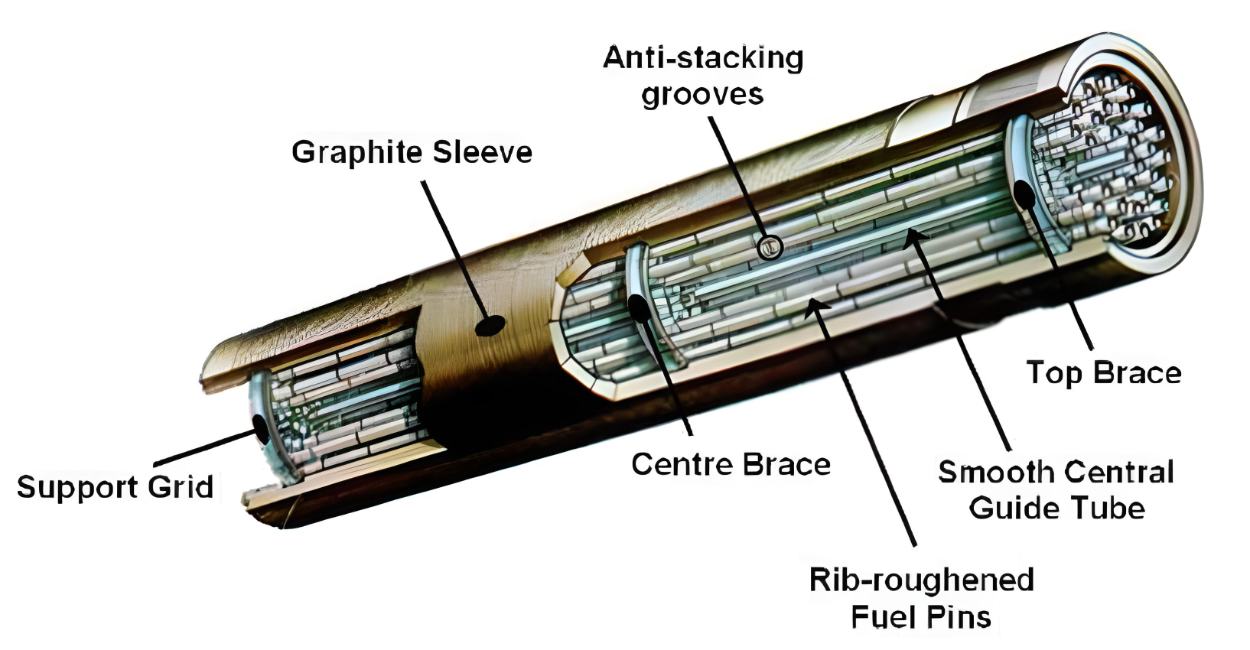
In full swing, the chain reaction heats the moderator water to dangerous levels. Control rods that absorb neutrons are not yet in place between the fuel rods.
In order to keep the chain reaction going, the neutrons generated by the fission process need to be slowed down by the moderator. The water, as a moderator, both acts as a coolant and a medium of friction.
To illustrate, here is a fission example: The uranium-235 core in the central fuel rod is broken apart by a slow neutron. This releases a burst of fast neutrons, which travel through the air before entering the water and being moderated by this medium. There is a significant chance that the slowed-down, thermal neutrons may break more uranium-235 nuclei.
Shutdown of the reactor by the control rods
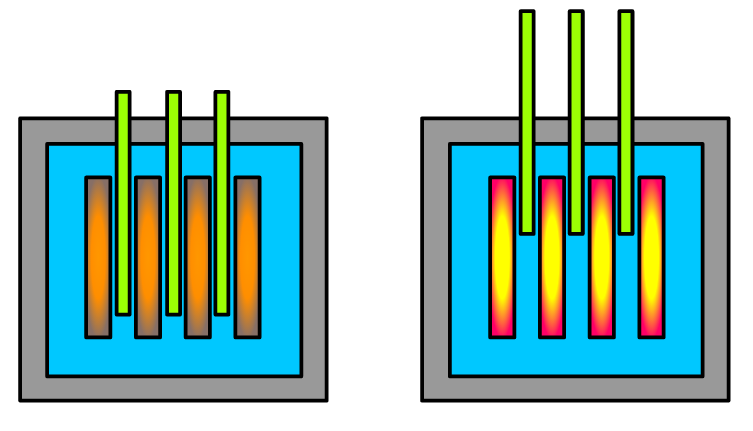
When the control rods are lowered, the reaction is subcritical, meaning that not enough neutrons are being absorbed to start a chain reaction. When the rods are withdrawn, the fission reactions speed up, the reactor enters a critical state, and the fuel rods begin to generate heat. As the temperature within the reactor rises, the control rods are put back into position to slow the ensuing chain reaction.
The chain reaction can be temporarily halted by moving neutron-absorbing control rods between the fuel rods. The moderator calms down as a consequence of the pause in the chain reaction.
Not all of the neutrons created during fission in the intermediate rod are accessible for fission reactions because they have been absorbed by the control rod. As a result, the reactions of fission weaken dramatically.
Bibliography
- Nuclear explained – U.S. Energy Information Administration (EIA).
- Plutonium – World Nuclear Association.
- Oklo: Natural Nuclear Reactors—Fact Sheet, Archive.
- History of the chain reaction in chemistry, NobelPrize.org.