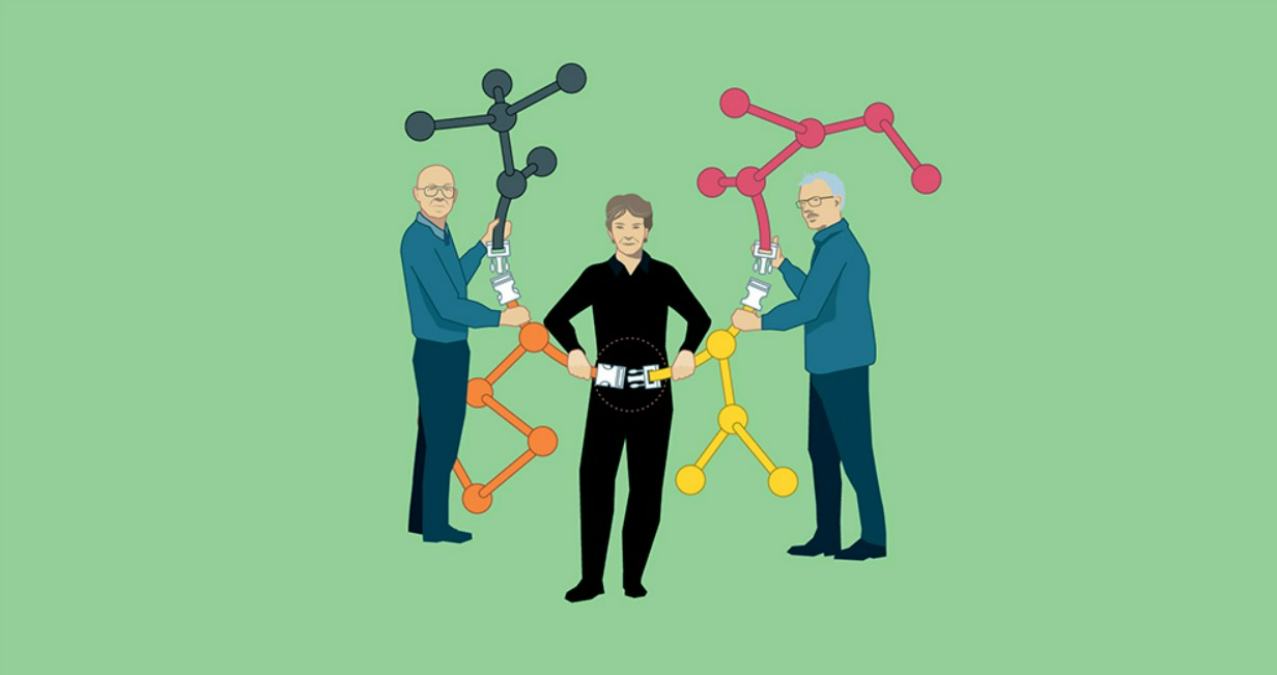
Three scientists will share the 2022 Nobel Prize in Chemistry for developing “click chemistry,” a modular synthesis in which standardized reactions can produce practically any organic compound from basic starting ingredients. It was American scientist Barry Sharpless who initially proposed the concept of click chemistry; Danish chemist Morten Meldal created one of the nuclear reactions for it, and American chemist Carolyn Bertozzi refined the techniques for application in live cells.
Thanks to the efforts of these two men and one woman, “click chemistry” can be used to synthesize organic compounds in a modular fashion.
Different molecules can be obtained by combining the elements of the periodic table through chemical reactions and chemical bonding. However, chemical synthesis, especially of complex compounds and active substances, frequently needs a series of consecutive reaction stages, each of which must be accomplished under certain circumstances. Because of this, many chemicals were produced way too slowly and inefficiently for a long time.
Three organic chemists will share the Nobel Prize in Chemistry in 2022 for their ground-breaking work in the field that has significantly streamlined chemical synthesis.
Barry Sharpless and molecule construction
A U.S. scientist named Barry Sharpless, who was awarded the Nobel Prize in Chemistry in 2001, is considered to be the catalyst for this whole endeavor. He was on the lookout for ways to streamline the molecular synthesis process at the time. His theory was that via modular synthesis, a large range of products might be obtained from basic starting ingredients. This could be accomplished by using a set of simple, generally applicable reactions as tools.
You might think of this method as being similar to Ikea’s, where you get a set of pre-fabricated, standardized parts along with some basic tools and put them together to make whatever shelves or cabinets you need. Sharpless and his colleagues characterized this concept in a 2001 technical publication as “clicking” modules together. That’s how Sharpless started the whole “Click Chemistry” thing.
In order to make their modular synthesis method as broadly applicable as possible, Sharpless and his team also established criteria for the reactions and starting materials involved. Specifically, they instructed chemical reactions to be occured in the presence of oxygen and water, and without the use of any special solvents. Further, the input and output materials should be conveniently accessible and separate as well. Theoretically, scientists have already suggested the first reactions that can be used in click chemistry.
Morten Meldal: The single most useful response mechanism for “clicking”
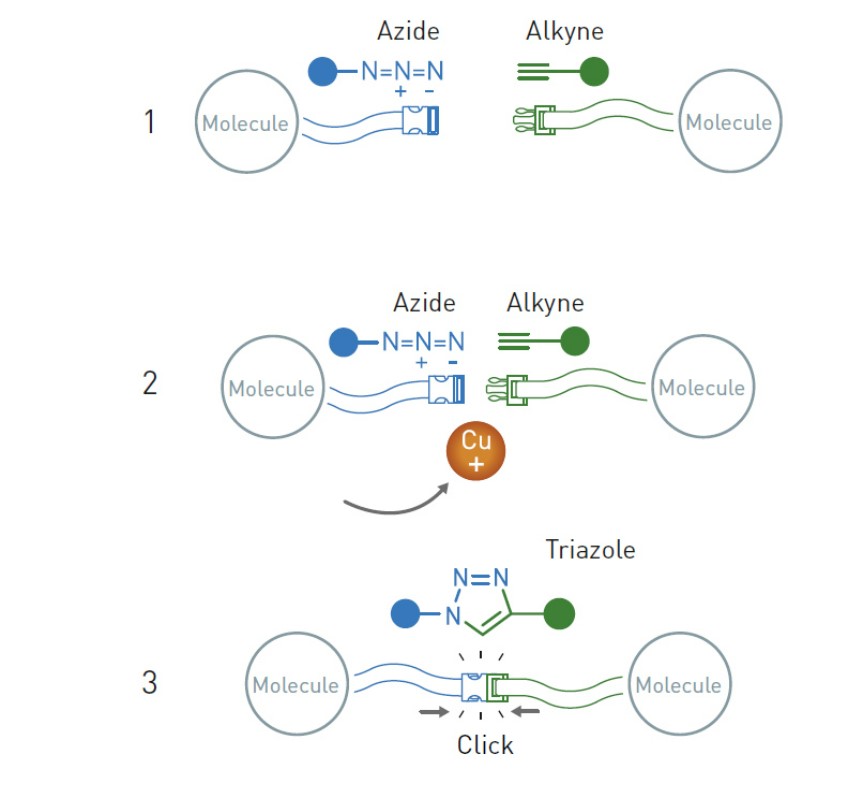
Second-place winner of the 2022 Chemistry Nobel Prize, Morten Meldal of Denmark presented one of the reactions mentioned by Sharpless and his colleagues at a conference the same year. Meldal discovered that the addition of copper to the azide-alkyne cycloaddition reaction in organic chemistry greatly improved its efficiency, eliminating the need for high temperatures while allowing the reaction to continue almost autonomously and with high yields.
The azine and alkyne groups in this copper-involved azide-alkyne cycloaddition combine like their click analogs. When attached to any organic molecule, they allow for the “clicking” of ever more complicated molecular structures. This reaction is now the cornerstone of chemical synthesis, which is employed in the creation of innumerable useful products in technology, medicine, and science, including medicines, plastics, and more.
Cellular click chemistry by Carolyn Bertozzi
(Bertoni et al. / PNAS 2007)
U.S. scientist Carolyn Bertozzi, who came in third place for the 2022 Chemistry Nobel Prize, expanded the use of this fundamental click chemical reaction to live cells, where it can be utilized to bind fluorescent marker proteins to biological components. However, copper is poisonous to cells, so she had to come up with a version of the azide-alkyne cycloaddition that works without this catalyst but still doesn’t need any more energy to proceed. The chemist accomplished this by using an alkyne version with a ring structure.
As an additional step, Bertozzi improved upon a well-established synthesis process called the Staudinger reaction to the point that it, too, could be used to “click together” the molecules in a cellular setting. This cellularly relevant click chemistry was dubbed “bioorthogonal reactions” by the chemist. She defines them as the interactions of functional groups that are sufficiently selective of each other that they may bind molecules together even in a highly dynamic and complicated biological context.
“The achievements and discoveries of Carolyn R. Bertozzi, Morten Meldal, and K. Barry Sharpless have had enormous influence on our society,” the Nobel Foundation stated. “Through the development of inspirational new concepts and highly efficient methods, the laureates have enhanced our capabilities and considerably deepened and widened our knowledge and understanding. Their remarkable accomplishments have increased our means to improve our world and better our lives, truly to the benefit of humankind.”