Who was Dmitri Ivanovich Mendeleev? All science students are familiar with the periodic table of chemical elements. The periodic table is a self-evident system: which way other than atomic weights could be used to arrange the elements? However, the origin of the periodic table is not at all simple; it required the synthesis of large amounts of fragmented and often faulty chemical and physical data into a stable system. Because of this, many scientists prefer to call it the “periodic law” to show how complex the network of relationships is, which includes how the elements in the periodic table are arranged.
In the 1860s, a few scientists were looking for a manual solution to the problem of arranging elements into a kind of table. However, many academics accept Dmitri Ivanovich Mendeleev’s work as the first successful system created in this field, which was announced in 1869, and they say he needed many more years to perfect his table.
Who was Dmitri Mendeleev?

Mendeleev was born in a small town called Tobolsk in western Siberia, Russia. His father was the principal at a high school there, but in 1834, the year that Dmitri was born, he had to retire for health reasons with an inadequate pension. This put the family’s livelihood on the shoulders of his mother, who was from a family of Siberian merchants. His mother had to operate a glass factory inherited by her near Tobolsk to support his family. However, the family’s financial situation deteriorated gradually.
After his father’s death in 1847 and the factory burning in 1848, when Dmitri graduated from high school in 1849, his mother decided to accompany him in his admission to the university, first in Moscow and then in San Petersburg, but his efforts were futile. Dmitri Mendeleev enrolled in the St. Petersburg Main Pedagogical Institute in 1850, where his father graduated years ago. Shortly after starting his education, his mother died, but Mendeleev managed to continue her education and graduated in 1855. After a short time, after teaching at secondary schools in the south of Russia, he returned to Saint Petersburg and started his postgraduate education in chemistry.
In his early scientific studies, Mendeleev gained extensive knowledge about the chemical properties of elements and many compositions. His first published work looked at the relationship between crystals and their chemical composition. The master’s thesis investigated the existence of a relationship between the chemical composition and crystallographic forms of specific volumes of the compounds.
In 1859, Mendeleev went on a long study trip with a state scholarship. He traveled all over Europe but spent most of his time in Heidelberg, Germany, conducting original research for his doctoral degree. He attended the first international congress of chemists, called the Karlsruhe Congress, which helped standardize many different chemical concepts, such as atomic weight and valence, held in Karlsruhe in 1860. The conference had a profound effect on Mendeleev’s way of thinking.
He later helped in the approval or procurement of conditions (especially the standardization of atomic weights) that would prove to be important for the development of the Periodic Law. That also had a stimulating effect on how other scientists began developing tables to organize the elements. In the 1860s, many different element tables were proposed, the most notable of which was presented by Lothar Meyer and John Newlands.
When he returned to Russia in 1861, Mendeleev taught chemistry in various educational institutions and gradually worked on his doctoral thesis. Also, he published various works on chemistry. When he received his doctorate in 1865, he became a professor at the most prestigious university in the country, Saint Petersburg State University.
Systematize the elements
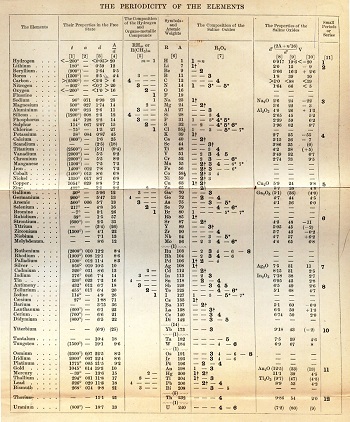
Mendeleev found the existing textbooks for chemistry in 1867 inadequate and decided to write one himself. This decision would be decisive in the discovery of the Periodic Law. Mendeleev decided to organize a large amount of chemical data in a useful and convenient way for pedagogical practice. Textbooks at the time dealt with the elements in dictionary style, just like metals and non-metals, by dividing them into general categories. Mendeleev sought a more suitable method.
He started his book, titled Principles of Chemistry, by evaluating the basic chemistry definitions in a wide range, along with the laboratory experiments that the students will do. From there, he switched to more common compounds and elements, such as salt, oxygen, carbon, nitrogen, and hydrogen. At this point, he realized that he would need another arrangement method for other elements, probably in late 1868 or early 1869.
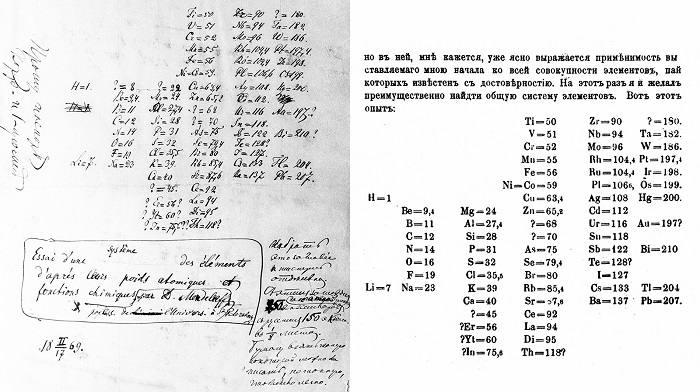
He tried to take the atomic weight as the primary attribute for each element, which shortly after led him to the idea of the periodicity of the elements. He quickly obtained the preliminary result and published it in a Russian periodical magazine after a short presentation at a meeting of the Russian Chemical Society. Most people think that Mendeleev came up with the Periodic Law after having a dream on February 17, 1869. However, it seems much more likely that he wrote the book about the Periodic Law after a long period of elaborate thinking.
Mendeleev had developed the essence of the system but still needed to consolidate it with detailed chemical and physical data showing the periodic properties of the elements. For nearly two years after its publication in 1869, Mendeleev worked hard to support his initial understanding with extensive chemical and physical data derived from his own experiments and extensive research in scientific literature. He was looking for a “natural system” in which the properties of each element would be periodically related to those surrounding him in the table. At the end of 1871, Mendeleev was confident enough to publish his results in a distinguished German scientific publication with a lengthy article. He envisaged various chemical and physical properties of the elements.
Mendeleev’s original publication of the Periodic Law attracted very little attention, except for a handful of scientists working for the same purpose. However, after 1875, and especially in the 1880s, this indifference began to change. The main reason for this was the discovery of some new elements with properties that closely match the characteristics of unknown elements envisaged by Mendeleev. In 1875, the new element gallium was discovered by the French chemist Paul Emile Lecoq de Boisbaudran. Mendeleev saw that the properties of gallium soon coincided with those of one of his elements. In 1879, Swedish chemist Lars Fredrik Nilson discovered scandium, and he said how compatible it was with Mendeleev’s predictions.
Many scientists declared that the periodic table fits the properties of the newly discovered elements as well as the known elements. In 1886, German chemist Clemens Alexander Winkler discovered germanium, and once again its properties were in harmony with Mendeleev’s predictions. The Periodic Law was on the way to becoming a widely accepted scientific principle. However, Mendeleev had priority quarrels with some scientists, especially Meyer, after the presentation of the Periodic Law. However, thanks to his fiery and resilient personality, he was the main explorer of the Periodic Table in the eyes of most people with whom he had close contact.
Mendeleev pursued a privileged career after his discovery of the Periodic Law. In addition to teaching and doing scientific research activities, he actively advised the Russian government and the private sector on many different economic issues, and he completed his professional life with the management of the Office of Weights and Measures. Dmitri Mendeleev became the icon of Russian science. He was recognized throughout Russia as a leading example of Russian scientific heroism.
Dmitri Mendeleev quotes
“There is nothing in this world that I fear to say.”
“I saw in a dream a table where all the elements fell into place as required. Awakening, I immediately wrote it down on a piece of paper.”
“Work, look for peace and calm in work; you will find it nowhere else.”
“It is the function of science to discover the existence of a general reign of order in nature and to find the causes governing this order. And this refers in equal measure to the relations of man – social and political – and to the entire universe as a whole.”
Bibliography:
- Strathern, Paul (2001). Mendeleyev’s Dream: The Quest For the Elements. New York: St Martins Press. ISBN 978-0-241-14065-9.
- Mendeleev, Dmitrii Ivanovich (1901). Principles of Chemistry. New York: Collier.
- Elena Konovalova (2006). A Book of the Tobolsk Governance. 1790–1917. Novosibirsk: State Public Scientific Technological Library, 528-page, p. 15 (in Russian) ISBN 5-94560-116-0
- Myron E. Sharpe, (1967). “Soviet Psychology”. Volume 5, p. 30.
- “A brief history of the development of the period table“, wou.edu
- John W. Moore; Conrad L. Stanitski; Peter C. Jurs (2007). Chemistry: The Molecular Science, Volume 1. ISBN 978-0-495-11598-4.