Ethyl acetate, a common solvent, may now be produced from organic whey wastes, negating the need for oil and natural gas in the production process. Creating the ethyl acetate solvent from milk processing leftovers in a sustainable way is crucial as it is used in adhesives, printing inks, or coatings. This technique is now possible thanks to a specialized membrane. Additionally, the whey molasses, which was previously problematic to dispose of, may now be recycled in a sustainable and practical manner.
Every day, the dairy industry generates a massive amount of whey as a byproduct. 2.2 pounds or 1 kilogram of cheese results in 20 pounds or 9 kilograms of whey being generated. That amounts to over 14 million US tons or 12.5 million metric tons of this liquid every year in developed countries. Some of it is turned into whey drinks with various fruit and other flavorings. Whey’s lactose and proteins may be extracted for further use as basic materials in medicines or infant formula.
But after the proteins and lactose have been isolated, you’re left with molasses. Because of its relatively high salt content, getting rid of this molasses has been a huge hassle and expense, up until recently.
Obtained from whey molasses instead of oil
Scientists from the Dresden University of Technology and the Fraunhofer IKTS have devised a method to extract valuable ethyl acetate from molasses. Surfaces cleaned using this colorless solvent are also used in the creation of adhesives, printing inks, and varnishes. For the longest time, ethyl acetate was made from natural gas and petroleum byproducts.
Now, ethyl acetate made from whey might serve as a more eco-friendly and long-lasting option. Unlike many common solvents, organic ethyl acetate breaks down rapidly in the presence of microorganisms, and it doesn’t rely on the increasingly precious resources of gas and oil. Additionally, this ethyl acetate extraction method provides a means of recycling whey molasses, therefore avoiding the high costs associated with its former disposal.
A composite membrane for the separation
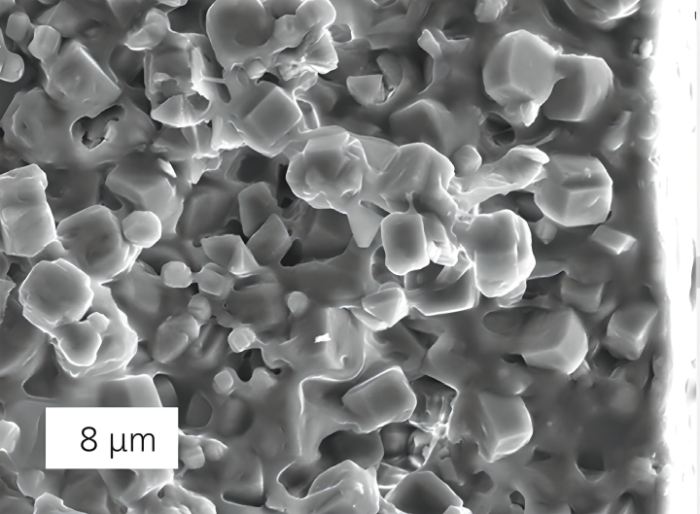
Ethyl acetate is extracted from whey in two steps. The first stage involves aerobic fermentation of the molasses in a bioreactor. A gas-steam combination containing ethyl acetate is created as a result of it. Next, special composite membranes are used to segregate the components. Scientists used a straightforward, low-cost method to create a membrane with very microscopic holes.
The membrane is made from a crystalline, porous alumino-silicate compound called inorganic zeolite. The membrane’s total thickness is about 10 micrometers, and its pores are only 0.5 nanometers in width. The chemical reaction between zeolite and ethyl acetate results in the separation of ethyl acetate and whey. Adsorbed by the zeolite, the molecules then diffuse through the composite membrane as they move along the pore surfaces. And, unlike the traditional method, high pressure is not required for this.
No polluting leftovers, high purity
The ethyl acetate purified from the whey molasses using such membranes has a purity of 97.5 percent. This eliminates the need for further processing procedures, conserving both energy and raw materials, and allowing for rapid usage as a raw material. According to the team, there are no adverse effects on the environment from discharging the gas/water vapor combination that is produced as a byproduct.
The study’s authors claim that their method could be used for more than only obtaining ethyl acetate from molasses. The method could be useful for separating gas mixtures or other substances with high volatility, including hydrocarbons.








