Complex interactions between a metal’s electrons determine how conductive it is. But thus far, they have only been dimly explained. Amazingly easy to understand, scientists have discovered a mathematical formula that accurately expresses the conductivity of all metals as a function of temperature. Even the exotics in this group of elements can now be classified consistently using this formula. The formula also sheds fresh light on the processes that underlie conductivity and temperature-dependent subatomic interactions.
Most of the time, metals exhibit conductivity toward heat and electricity. The atomic structure of certain atoms enables this. The outer electrons of the metal atoms are mobile in parts and form a “lake” of electrons around the atomic bodies. This indicates that there are sufficient charge carriers available to support the current flow. However, every one of them has a different level of resistance and a different response to heat.
Only partly known mechanisms
Yet, the processes behind the conductivity behavior of metals are still poorly understood. There is still a lack of a standard definition of metallic resistance. Thus, there has never been a formal categorization system. In terms of conductivity, physicists refer to “normal,” “strange,” “Planckian,” or even “correlated” metals.
These metal groups have different electron interactions, which affects how responsive their conductivity is to temperature changes. For instance, in “normal” metals, repulsion effects make sure that the electrons rarely interact and that the resistance varies roughly linearly with temperature. On the other hand, correlated metals have strong electron-electron interactions that, beyond a particular temperature, can transform these substances into superconductors.
But, these various metal groupings have not yet been routinely or properly characterized. The researchers decided to examine more metals in more detail. The team examined and contrasted the temperature-dependent resistivity changes in 30 metallic elements for their study.
One formula to accommodate all the information
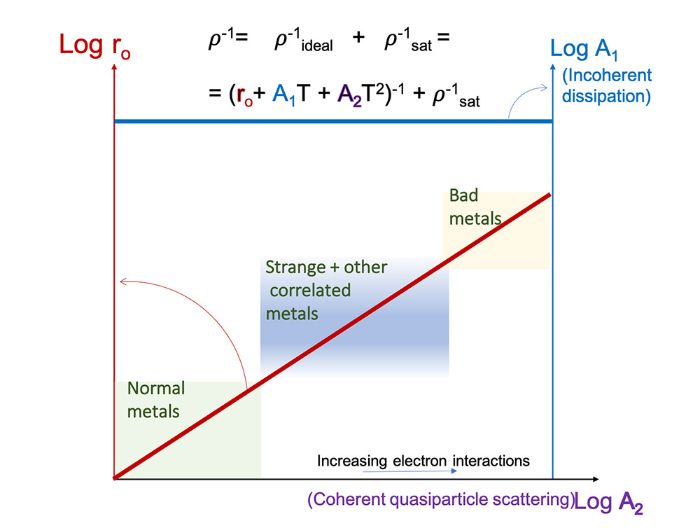
In the process, the researchers made an unexpected discovery: regardless of whether a metal was labeled as normal, correlated, or strange, they discovered startling agreement when they sought to characterize the behaviors of various kinds of metals to growing temperature in mathematical-physical terms. All of the data sets could actually be described using the same kind of formula, which was a “Taylor series.”
Although there are distinct physical principles involved depending on the kind of metal, a uniform formula can now be used to explain the conductivity of metals at various temperatures.
To that end, the scientists were able to write the metal’s electrical resistance as a power series: r = r 0 + A 1 T + A 2 T 2 + A 3 T 3 … . T represents temperature, r represents resistance, while r0 and A represent separate constants. This formula is a mathematical description that relies just on two inputs; no physical assumptions are used in its derivation. However, the investigation revealed that this linear and quadratic formula actually applies to all metals.
A universal correlation
Finally, a formula has been found that describes the temperature dependence of conductivity for all metals, regardless of whether they are normal, correlated, strange, Planckian, or any other kind.
The surprising thing was that although these classes were based on different forms of electron interaction, a universal linear relationship emerged that was valid across all classes of metal.
Because the equation represents merely the reaction as such, the independent nature of the physical mechanism now becomes visible.
The formula depicts the proportional shift between linear and nonlinear dispersion in metals as the temperature rises and falls. It makes no difference whether this is caused by electrons interacting with one another or with wave phenomena like phonons. Additionally, the formula has helped to verify a long-held belief. Since the Planck radiation can only be observed at very low temperatures, this implies that all metals must have some degree of it.