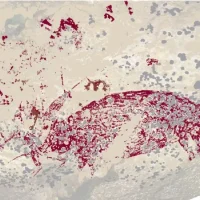
According to a new study, the active ingredient semaglutide in the coveted weight loss injection Wegovy and the diabetes medication Ozempic from Novo Nordisk may be linked to a dangerous eye disease. After analyzing data from over 16,800 patients at a large eye clinic in Boston, an international team of researchers concluded that people taking semaglutide have a four-fold increased risk of non-arteritic anterior ischemic optic neuropathy (nAION).
This eye condition results in sudden unilateral loss of vision due to a circulatory disorder of the optic nerve.
Diabetics have an increased risk for this disease. According to the study published in the journal “JAMA Ophthalmology,” among the patients, there were 710 people with type 2 diabetes and 979 people with overweight or obesity who were treated with either semaglutide or another medication. During the three-year observation period, the risk of a nAION diagnosis for diabetics taking semaglutide was 8.9 percent, compared to a risk of 1.8 percent when using other antidiabetic drugs.
For overweight and obese individuals in the semaglutide group, the risk was 6.7 percent, compared to 0.8 percent in the group taking other weight-reduction medications.
The scientists emphasized that this does not prove that semaglutide is the reason for this increased risk. However, further investigation of the connection is necessary. Medical professor Graham McGeown from Queen’s University in Belfast explained that while the study had a high quality level, the study authors themselves had pointed out important weaknesses.
Cause and Effect Unclear
The data doesn’t disclose the rationale behind administering the medications. A factor could be that those treated with semaglutide had previously been more severely ill with diabetes than the other subjects. Additionally, there are doubts about how representative the group of patients is for the US population. The semaglutide group was, on average, older than the others. This could explain the NAION risk, and people of African American descent are also considered disproportionately at risk.
Larger studies would need to verify the connection. “Given the rapid increase in the use of semaglutide and its potential approval for a range of problems other than obesity and type 2 diabetes, this question deserves further investigation,” McGeown stated.
Semaglutide belongs to the class of so-called GLP-1 agonists, which lower blood sugar levels and reduce appetite. Ozempic and Wegovy helped the Danish pharmaceutical manufacturer Novo Nordisk achieve record sales and become Europe’s most valuable publicly listed company. Novo stated that patient safety is the company’s highest priority. “We take all reports of adverse events associated with the use of our medicines very seriously.” Overall, however, the published data are not sufficient to establish a causal relationship between the use of GLP-agonists and nAION. There are also important methodological limitations to the study that must be considered when interpreting the results.
nAION occurs in about two to ten cases per 100,000 people, making it the second most common cause of blindness due to optic nerve damage. A previous study had already shown that semaglutide can worsen diabetic retinopathy, a disease of the retina. Therefore, specialists recommend an eye examination before and during semaglutide use.