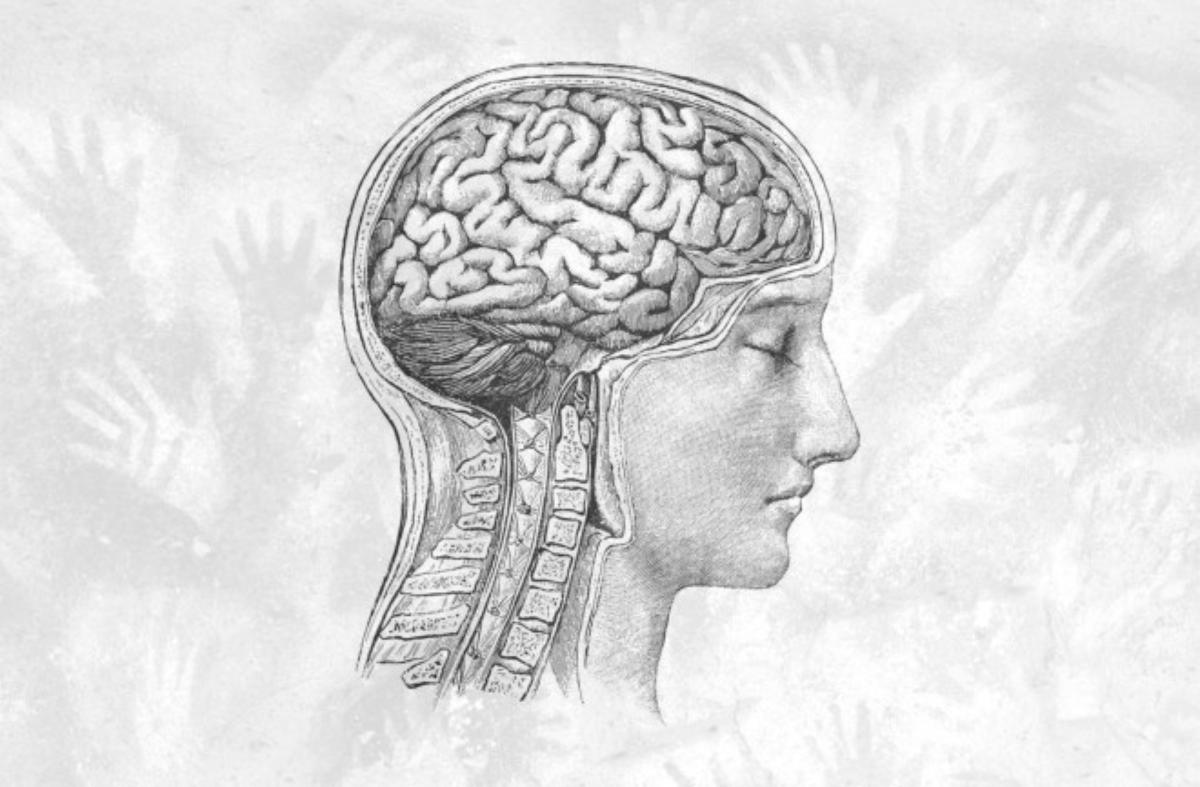
Anyone suffering from migraines has undoubtedly heard the term “trigeminal nerve” from their doctor. This comes up when physicians explain how the agonizing, persistent pain in the head occurs. The trigeminus is a cranial nerve that connects the forehead, face, and chewing muscles to the brain. It sends faulty signals to blood vessels in the meninges, according to the currently prevailing theory. This is supposed to release inflammatory substances that cause the vessels to dilate, become more permeable, and thus begin the vicious cycle of pain.
New data from the United States now challenges this idea: While they also attribute a prominent role to the trigeminus in migraines, researchers led by neurobiologist Maiken Nedergaard from the University of Rochester, New York, have managed to show that it’s not about misdirected nerve signals. They demonstrate something previously considered far-fetched: In migraine patients, the trigeminal nerve has a hole at its brain-side end.
The work was published in the journal Science. The hole is located between the trigeminal nerve cells that process signals from the face and the brain. More precisely, its membrane. This allows cerebrospinal fluid to reach the cluster of nerve cells. This explains why a pain attack follows a migraine aura.
A migraine aura can be auditory hallucinations, numbness, but most often visual disturbances, temporary blindness, flashes of light, blinding circles, or “shooting stars.” These symptoms typically occur five to 60 minutes before the headache. It’s estimated that one in 10 people suffers from migraines, and in about a quarter of these cases, the headaches are preceded by an aura.
During a Migraine, There’s a Wave in the Head
At that moment, a loss of voltage occurs at the level of brain cells. The cells, which normally build up electrical voltages across their membranes, “depolarize.” This means they can no longer build up electrical voltage and, thus, can no longer communicate. Most frequently, the depolarization event occurs in the visual processing center of the cerebral cortex, resulting in the visual symptoms that first announce an impending headache.
The substances that enable the voltage difference literally wash away, leading to the loss of voltage.
Nedergaard and her colleagues at the University of Rochester and the University of Copenhagen are pioneers in understanding fluid flow in the brain. In 2012, their laboratory was the first to describe the “glymphatic system”: Both the brain and spinal cord are surrounded by fluid-filled sacs. In emergencies, the system uses this fluid to flush out toxic proteins in the brain.
This also occurs spontaneously in migraine patients. In the process, proteins are released again as alarm signals, indicating that the voltage situation isn’t working properly.
Previously, it was assumed that the end of the trigeminal nerve rests outside these membranes, and the biological barrier there strictly controls which molecules can enter and leave the brain.
However, in experiments with mice where they artificially generated the fluid wave, the researchers found a previously unknown gap in the barrier. Through this, fluid could escape from the brain and flow directly to the nerve cell node at the tip of the trigeminus.
The nerves would then be exposed to a cocktail of alarm proteins.
The brain itself cannot generate pain when something is wrong. The trigeminal nerve can. And it’s precisely with pain that it reacts to the wave of fluid from the brain.
The discovery of alarm proteins is also somewhat novel. In total, they identified twelve proteins whose concentration increases sharply during a depolarization event and which simultaneously bind to receptors on the sensory nerves in the trigeminal end. A new class of medications already targets Calcitonin Gene-Related Peptide (CGRP), one of the proteins. These CGRP inhibitors prevent migraine attacks in advance.
Others of the alarm proteins identified in the Rochester study are already known from other pain studies. They suffer from neuropathic pain. Nedergaard and her colleagues now anticipate the development of effective pain blockers at this stage. They should prevent the proteins from triggering a pain signal at the trigeminus.
“In this study, we describe how the central and peripheral nervous systems interact with each other in migraine,” Nedergaard says in a press release from her university. “These findings provide us with many new targets for medications. We can devise strategies to avert nerve activation or migraine altogether.
The connection with the fluid wave in the head also explains why so many migraine patients have unilateral pain: There is one trigeminal end on each side of the head. The researchers observed that the transport of proteins released on one side of the brain primarily reaches the nerves on the same side.