Marilyn Monroe famously sang, “Diamonds are a girl’s best friend.” Not only do those who like flashy jewelry have a great deal of respect for diamonds, but so do scientists, engineers, and the makers of equipment. This is due to the fact that diamond is the hardest material of all. When pressed against another substance, the diamond will always triumph. This is why diamond dust is sometimes used to coat instruments like grinding wheels. But how does a diamond get to be so incredibly tough?
Diamond has a rigid, three-dimensional structure made up of perfectly similar atoms bonded together in perfect alignment. The only element present in diamonds is carbon. Each atom in this structure is bound to four others in a precise manner, forming a three-dimensional lattice. This lattice also provides tremendous resistance to any kind of rearrangement.
Nothing can move an atom from its position
How easily individual atoms or large clusters of atoms inside a material can be moved against one another is a primary factor in determining that substance’s hardness on the subatomic level. If the material provides a lot of resistance to the structural change, it’s considered “hard.”
Almost no atomic rearranging of a diamond is possible. All four of a carbon atom’s neighbors have the same amount of space between them since it is precisely determined where the bonds and the atoms are located in a diamond.
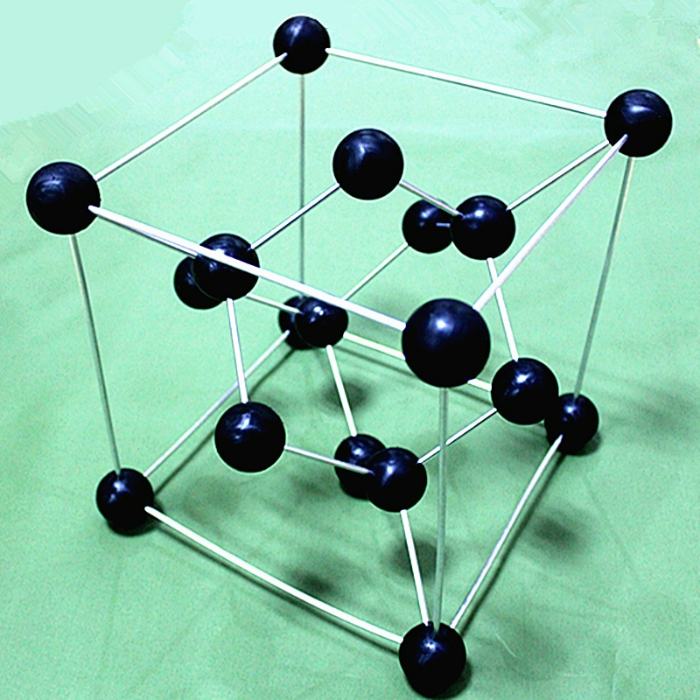
No matter where you look, the angle between any pair of nearby atoms in a diamond is 109 degrees. You just can’t change the angles to move the diamond atoms against one another by applying pressure on the material. That’s what makes the bonds in a diamond so stiff.
There has to be a full rupture of a carbon-carbon bond for it to happen. But it takes a tremendous amount of power, and the diamond no longer becomes a diamond once this bond is broken.
What about graphite?
Graphite, from pencil lead, an unusually soft substance, is the diamond’s closest cousin. Similarly, this is made up entirely of carbon. Yet, in graphite, the atoms have a layered arrangement. The graphite atoms are securely held together inside the layers, yet there are very mild forces between the layers themselves. Because of this, they may be displaced against one another with the gentle pressure of a pencil. Then, a few of the layers fall off and settle on the paper below. Because of this, graphite makes a great lubricant as well.
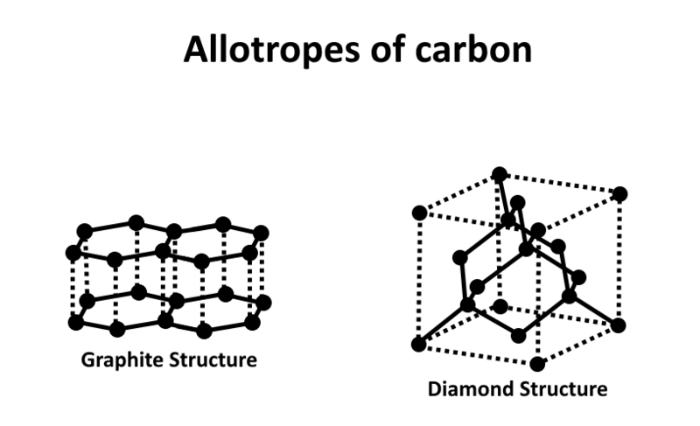
Only under extreme conditions of high pressure, such as those found at tremendous depths, could diamonds form naturally. If exposed to the atmosphere at Earth’s surface, they would actually change into graphite under low pressure, which is a more stable form.
This, however, takes place at such a snail’s pace that it is impossible to track. You shouldn’t put your diamond ring in the oven since this process accelerates at higher temperatures. The diamond will burn like coal if you put a flame on it. Even though diamonds are durable and reliable, they are not as hard to break as some marketers would have us believe.