From where did we come, and how did we evolve? A variety of scientific fields are interested in these issues, and they have no way to find answers without reliable dating methods. This is why scientists use a wide range of geological and archaeological “clocks” to date objects. The adjectives “old” and “young” in Earth’s historical context refer to periods of thousands to millions of years rather than the average human lifespan. Geochronology, the study of the absolute ages of geological and archaeological objects such as rocks, minerals, wood, and human remains, has developed various techniques for that. It has greatly influenced our contemporary worldview by revolutionizing our knowledge of Earth’s past.
Radiocarbon dating method
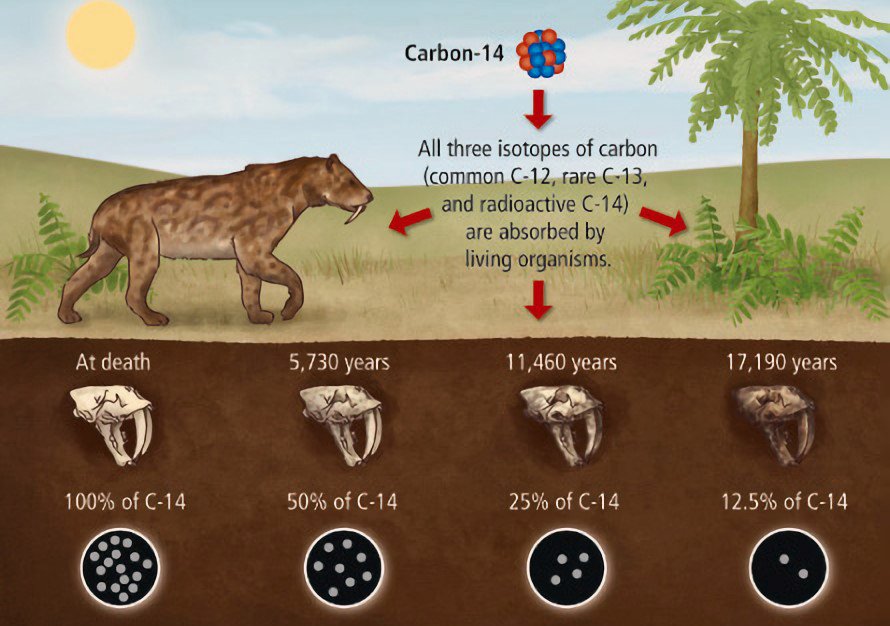
The so-called radiocarbon method, sometimes termed C14 carbon dating, is perhaps the most well-known technique for estimating age. Because carbon has three naturally occurring isotopes (C12, C13, and C14). There is just one carbon nucleus (C14) with 14 nucleons (6 protons and 8 neutrons) for every trillion of the stable C12 with 12 nucleons in the nucleus. The decay of the C14 isotope to nitrogen has a half-life of approximately 5,730 years.
The lower the amount of C14 isotope, the older the object.
All living things maintain a consistent C12:C14 ratio because they continually breathe in newly generated C14 from the environment. But C14 is not replenished if the animal or plant dies. Since the ratio of the two carbon isotopes in its tissues gradually becomes more favorable to C12 over time, this process provides the foundation for the radiocarbon clock. Wood, fossilized plant material, and bone can all be dated by analyzing their isotope ratios to determine how long they have been out of contact with C14. Similar techniques can be used to date other carbon-based substances.
A historical glance
The C14 clock’s main benefit is that compounds containing carbon are prevalent in both living and nonliving materials. C14 dating can be used in archaeology and paleontology to determine dates ranging from 300 to 60,000 years. But carbon mostly only exists in organic material.
It was in 1934 that Franz Kurie first proposed the possible existence of carbon-14. In 1940, at the University of California Radiation Laboratory, Martin Kamen and Sam Ruben made the discovery of carbon-14. In 1946, Willard Libby developed radiocarbon dating based on carbon-14 to determine the age of artifacts, rocks, and even water.
There were only a few institutions in the 1950s and 1960s responsible for the development of the C14 detection technique, which employed counting tubes to measure the decay of the carbon isotope and made it available for use in archaeology for the purpose of dating. This was the first time that archaeologists had used a scientific technique of dating.
Radiocarbon dating has allowed for a comprehensive study of the last 50,000 years of human history, shedding light on questions such as when Neanderthals went extinct in Europe and how the ancestors of modern humans spread in terms of their physical characteristics. However, this method of dating has also illuminated the end of the last ice age and the beginning of the present warm period, the change from nomadic to settled agriculture, and the first use of metals.
Eruption of a volcano
C14 dating has become standard practice in the field of archaeology. One of the many unexpected findings made possible by the C14 approach is the recalculation of the timing of a Late Bronze Age volcanic eruption on Santorini, which happened in the Aegean. Based on a combination of dynastic chronicle research and astronomical evidence, Egypt’s historical chronology places the eruption of the volcano in 1520 BC. Objects that survived the volcanic eruption and were buried in ash and rocks were analyzed using the C14 technique, which led researchers to infer that the eruption happened one hundred years earlier than previously thought.
Groundwater radiocarbon dating is another use of the C14 method. Using it, researchers can distinguish between glacial reserves and recently developed bodies of water in North Africa, where people rely on underground water supplies for drinking and other uses. Everyday applications of the C14 approach include verifying the authenticity of artifacts, determining the age of wines and spirits, and finding synthetic ingredients in purportedly organic items.
Zircon dating method
When did the Earth form? Because it only accounts for events that occurred during the last few thousand years on Earth, the radiocarbon approach is insufficient to provide an answer. But then, how can you put a time stamp on something as basic as Earth’s creation? Amazingly, geoscientists have realized that looking at the microcosm and focusing on the microminerals inside rocks is the greatest way to get an answer to this issue. From this investigation, a picture can be constructed of the progression and chronology of Earth’s history on a global and continental scale.
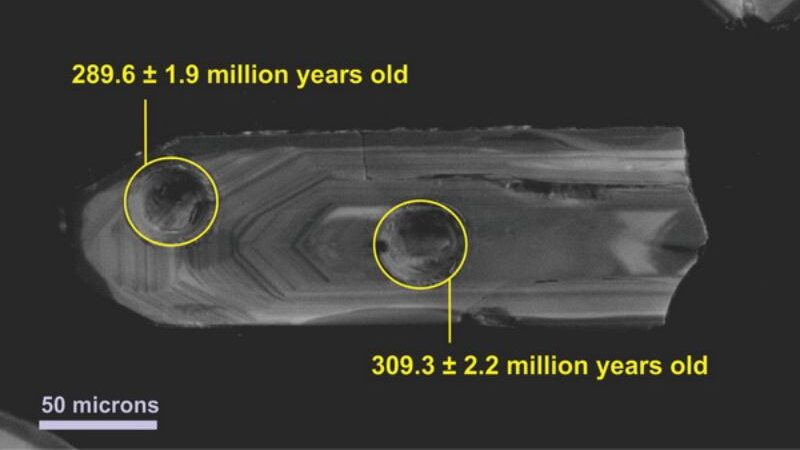
Zircon, known as the “star mineral” for its importance in reconstructing Earth’s past, is one of these little time capsules. The zirconium, silicon, and oxygen compound (zirconium silicate (ZrSiO4)) existed for nearly 4.4 billion years, making it the oldest mineral on Earth. Zircon can be found in abundance in the Earth’s crust. Typically measuring between 0.05 and 0.3 microns in size, it is found as minute grains in the rocks.
Uranium atoms encased in a crystalline structure
The presence of uranium in zircon’s crystal structure is an attractive trait for dating purposes. Since uranium decays into lead over time, scientists can use the decay rate to determine the age of an object by measuring the uranium/lead ratio. Uranium decay products are trapped in the crystal lattice of surrounding zircon to varying degrees depending on when the zircon formed.
Zircon can be used as a “clock” that can measure up to 4.4 billion years into the past, making it more accurate than the radiocarbon approach. Consequently, this technique works very well for dating rocks. Due to the very lengthy half-lives of uranium’s decay to lead, it cannot be used for this purpose over timescales of less than around 1 million years.
Zircon aids in the study of continental prehistory
How the continents were first created and how they eventually developed is a fundamental subject for Earth historians. In modern times, we have learned that the continents on Earth’s surface move gently in a back-and-forth motion. They could run against one another or shatter into pieces. However, the age of the various parts of the continents varies widely. Each continent is really made up of several other, smaller continents called “terranes” that range in age from very young to very old. There is a unique signature of time for each terrane. But how can we figure out the age distribution of each one?
Gondwana and Pangea
In order to address this issue, geoscientists analyze zircon for the uranium and lead isotopes it contains. This technique of dating has been utilized to investigate several aspects of crustal rocks. Crusts have a complicated history. 550–500 million years ago, it started on the northern margin of Gondwana, a prehistoric continent in the southern hemisphere. A number of massive chunks separated off the primitive continent’s northern border. They moved up north as separate continents, eventually forming a complex mosaic.
About 350–330 million years ago, the core region of the newly developing primeval continent Pangea was where the jigsaw pieces were ultimately fused together. However, 120 million years ago, Pangea, the supercontinent, started to split apart into different continents. The Atlantic Ocean opened, Africa was divided from South America, and North America and Europe were separated from one another.
The zircon data show, for instance, that the European crust is made up of smaller continents that were still linked to modern-day North Africa 500 million years ago. Central Europe thus sits on a piece of the Earth’s crust that was once part of Africa many eons ago. For the record, Canada is home to the world’s oldest continental crust. Age estimates put it at upwards of 4 billion years. When compared, Europe is a very young continent.
Uranium dating method
When it comes to dating the rock, the potential mechanisms of uranium in minerals are considerable as well. In addition to dating the rock, they also show how its temperature has evolved through time. You can use this to figure out when lava solidified or how slowly a rock cooled.
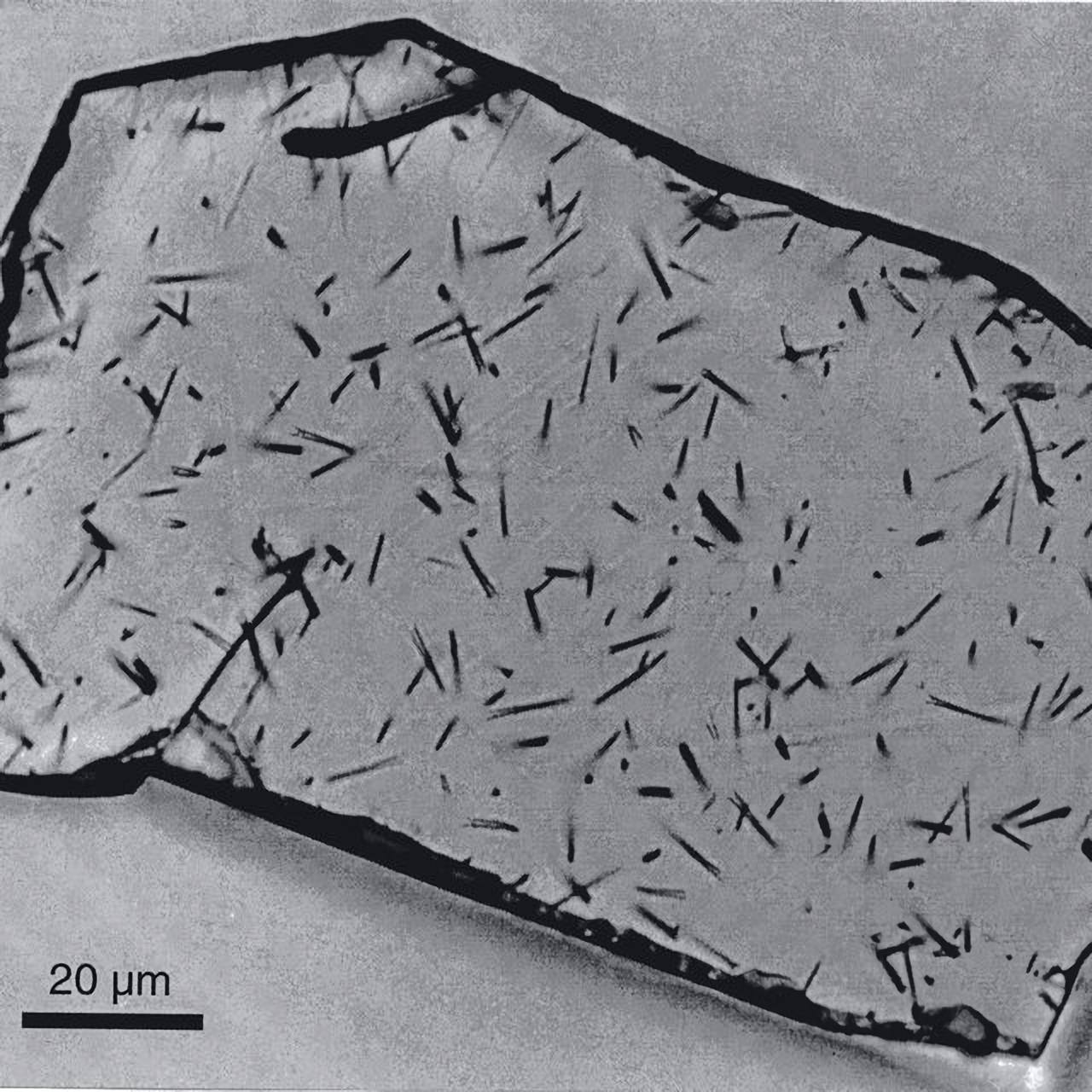
Everything functions on a very basic principle: Fission marks are tiny destructions caused by fission products blasted into the mineral lattice when a uranium nucleus contained inside the material decays. These fission markings, which appear as thin lines and notches if the mineral grain is polished, prepared in a certain manner, and seen under a microscope, are readily apparent. Since the rate of uranium decay is well established, scientists can determine the age of a mineral by counting the number of fission traces inside it. The greater the number of traces, the longer the material was exposed to radiation, and hence, the greater the amount of damage it suffered.
Intense heat causes a time reset
But there’s more to think about: These fission marks can only occur if the mineral isn’t overheated. Fission markings in the lattice of apatite, for instance, shorten if the temperature is over 60 degrees Celsius. Above 110 degrees, the crystal lattice heals, and the atoms that were displaced by the fission products return to their original lattice positions, leaving no evidence behind. The timer on the fission track has been turned back to zero.
The “clock,” which is dependent on the temperature of the rock, aids geologists in several ways: Fission traces cannot occur in the rock at these depths because the rock is too hot. If it is then hauled upward by plate tectonics, these traces will become visible. Geoscientists may calculate the time it took for a layer of rock to ascend from the depths of the Earth’s crust to the summit of a mountain range based on its present height, the known decay rate of uranium, and the quantity of fission traces it contains. For instance, scientists have calculated that the Val Bregaglia in Switzerland, is rising at a pace of 0.3 mm each year.
Ancient glass and erupting volcanoes
This approach can also be used in dating rocks: Lava often hardens within a short period of time after a volcanic explosion. Thus, the age of a volcanic mountain can be determined from the last eruption or formation of the corresponding volcanic rock by analyzing the fission residues in its minerals. This method can be used to date man-made objects as well. Glass was often dyed yellow using a uranium oxide compound from Roman times until well into the 19th century in Europe, North America, and China. Fission markings indicate the time the glass solidified, and can be used to date objects made of glass, such as drinking glasses or figurines.
Potassium-argon dating

Also known as K–Ar dating, the potassium-argon dating is a technique for establishing the age of rocks by analyzing the abundances of radioactive argon and potassium inside them. Radioactive potassium-40 decays to argon-40 in minerals and rocks, providing the basis for this dating technique.
But there is a similar decay process for potassium-40, which results in calcium-40. So, the age of a mineral or rock can be calculated from the proportion of these radioactive isotopes.
However, the widespread availability of nonradiogenic calcium in minerals and rocks makes the calcium-potassium age technique seldom used. The escape of argon to the atmosphere during volcanism, on the other hand, results in a relatively low argon abundance on Earth.
However, although radiocarbon dating can only identify an age up to around 60,000 years, potassium-argon dating can go back as far as about 100,000 years. As a result, a void between 60,000 and 100,000 years has been found, which must be bridged using other dating methods. Thermoluminescence dating covers this by going from 40,000 to 200,000 years ago.
Nature as a dating method
In addition to radiometric dating, geochronologists may use a variety of different techniques to determine when an object was formed. The foundation for this comes from the fact that many natural materials, like wood or sediments, document and archive temporal events and changes across time. The only thing we need to do is figure out how to read and accurately interpret nature’s time records.
Annual rings in wood and sediment

The seasons have an impact on the emergence of new geological structures or the deposition of new materials. For instance, during tree development, one can count the rings in the wood and, under ideal circumstances, estimate the tree’s age to within a year. Dendrochronology, the study of tree rings, has allowed researchers to construct chronologies for continents that go back some 14,000 years. Tree-ring dating, like carbon-14 dating, is an important tool in archaeology for establishing the age of structures like fortifications and habitations.
However, Varve chronology, also known as soil dating, examines the layers of sediments to determine when they were deposited. Lake sediment, like tree rings, is produced in seasonal cycles, with summer producing a slightly different composition and, generally, a different colored layer than winter. Therefore, the age of a sediment layer can be determined, as can the biological and climatic conditions that existed during a certain time period, by analyzing the sequence and thickness of these layers.
Depending on the specific circumstances, this method may provide an age estimate of between 40,000 and 70,000 years. The effects of historical climate change are typically inferred through varves.
Thermoluminescence dating

The term “thermoluminescence” is a combination of two ancient Greek and Latin words meaning thermos “hot” and lumen “light”. The term means “light from heat”.
Natural radioactive elements found in rocks and ceramics are used in various techniques to determine the age of rocks. Quartz and feldspar, for example, have electrons that get essentially imprisoned at particular disruptions in the crystal lattice due to the radiation released by these materials.
Electrons are affected by these disturbances in the same way that spheres are affected by depressions on a surface; they may roll into them but cannot roll out on their own. Therefore, the radioactive decay energy is still present in the materials. Light or heat releases the trapped electrons, which then rearrange themselves and release any extra energy as luminescence.
How much energy the radioactive inclusions have stored in the material can be deduced from how bright the luminescence radiation is. In this way, it can be used to date items to at least 100,000 years ago. Thermoluminescence and optically stimulated luminescence are two terms used to describe these techniques for determining the age of the rocks.
Thermoluminescence dating is advantageous because it can be used to date objects anywhere from 40,000 to 200,000 years ago, a time range that falls in between those of radiocarbon and potassium-argon dating.















