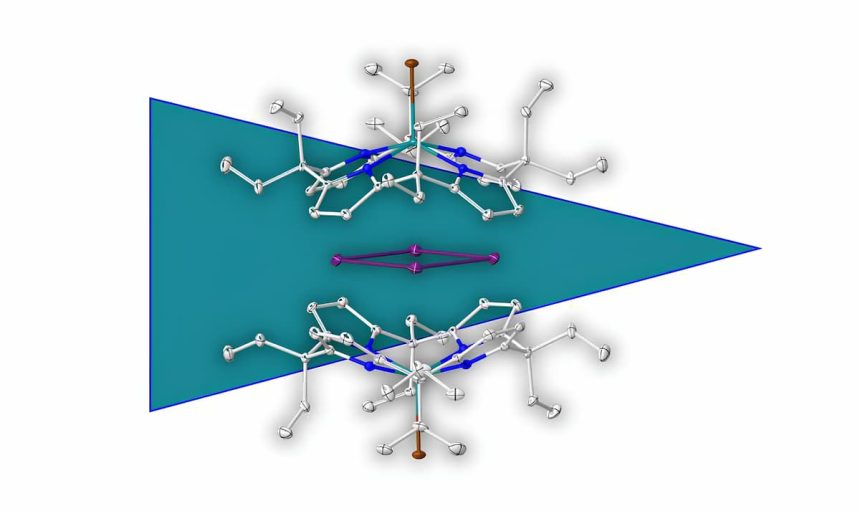
For the first time, chemists have succeeded in creating an aromatic ring compound consisting only of metal atoms without linked support molecules. The team reports in “Nature Chemistry” that the ring consists of four positively charged bismuth ions, connected to each other and with delocalized bonding electrons. A charge field of two unconnected but neighboring molecules stabilizes this ring compound.
Aromatic compounds are ring-shaped molecules with loosely assigned bonding electrons between the atoms. Instead, they form delocalized orbitals that extend around the entire ring or large parts of it. This gives them unique stability and properties. It’s no wonder, therefore, that an estimated two-thirds of all organic molecules are wholly or partially aromatic.
The framework of such a ring usually consists of carbon atoms, as in benzene, but it can also be formed by other atoms, such as nitrogen or metals. In the latter case, however, only aromatics are known so far in which the ring-shaped connected metal atoms are additionally stabilized by organic molecules covalently linked to them.
Four Metal Ions in the Aromatic Ring
But it’s possible without them, as chemists led by Ravi Yadav from the University of Heidelberg have now demonstrated. For the first time, they have succeeded in creating an aromatic ring consisting of positively charged metal ions without attached molecules. The ring consists of four bismuth cations (Bi+), whose total of 16 bonding electrons are delocalized. It is the first cationic, purely metallic aromatic compound without covalent “support molecules.”
However, this metallic aromatic ring is only possible because there are two larger organic molecules in its immediate vicinity. Although these are not connected to the metal ring, they still serve as stabilizers: “The highly electron-deficient ring is held in the symmetrical charge field formed by two electron-rich calix-pyrrolate units,” the chemists explain. Held by this charge field, the metal ring floats in a cavity between the two accompanying molecules.
Also Possible with Other Metals
Additional tests showed that other metals with similar outer electron configurations, such as lead and tellurium, can also form such cationic aromatic rings. In all cases studied, four-membered rings with delocalized sigma orbitals were formed, as the team reports. “We expect that our approach can be applied as a general method to other areas of stabilizing positively charged rings and cages,” says senior author Lutz Greb from the University of Heidelberg.
At the same time, their experiments also provided new insights into the bonding behavior of metal atoms. “Aromatic compounds made of pure metal atoms initially serve fundamental understanding. However, some unexpected effects in our work point to a new basic concept in the field of aromaticity,” explains Greb. “It could be significant for charge transport in metals.”