Not quite a solid and not quite a liquid, physicists have found that water can take on entirely new forms in controlled environments. This suggests that at ambient temperatures, a monolayer of water molecules may undergo a phase transition into the “hexatic” state, in which the H2O molecules spin in place while being neither solid nor liquid. Physicists report in Nature that at slightly higher pressures, liquid water becomes superionic, adopting a condition previously theorized exclusively for planetary nuclei.
The water is both familiar and foreign. The apparently straightforward molecule H2O really displays a wide variety of quirks even under normal conditions, including density anomalies, self-dissociation, and more than a dozen distinct ice forms. However, when the water molecules are substantially constricted, as in tiny veins, the phenomenon takes on an even more outlandish flavor: water abruptly loses its dipole moment and becomes “electrically dead,” and it also flows with less resistance than it normally would.
Confined water everywhere
Also, physicists from the University of Cambridge headed by Venkat Kapil found out that this isn’t all there is to it. Researchers have utilized sophisticated physics models and AI to examine the effects of temperature and pressure on the structure of a single layer of water. Nanoscale cavities being filled with water are crucial to the functioning of innumerable common events.
Such ultrathin water layers are present in the capillaries and membranes of all living beings, they are the defining feature of the pore water in subterranean rocks, and they play a vital role in many technological, medicinal, and chemical processes. The behavior of “restricted” water in a variety of environments is, nevertheless, poorly understood. The attraction forces of the “walls” are the only thing that can be said for definite when discussing the pressure exerted on water molecules in such tight spaces.
Phase diagram for single-layer water
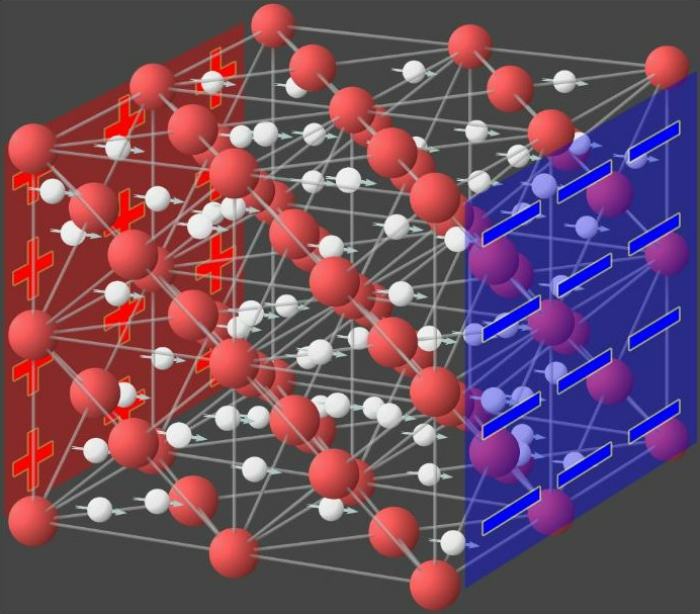
The analyses of Kapil and his colleagues have resulted in the first phase diagram for single-layer water, which depicts the changes in phase as a function of both temperature and pressure. The first-principles approach they used in their modeling successfully captured the state of single-layer water. This method relies only on established rules, which eliminates the possibility of erroneous conclusions being reached due to the introduction of unproven assumptions or guesses. This is considered the gold standard in the realm of physical modeling and computation.
The analysis began by confirming several previously known phase transitions, such as the single-layer water undergoing a series of ice phases when cooled to temperatures below freezing. Under increased pressure, the water molecules rearrange themselves from the standard hexagonal atomic lattice to a pentagonal form, a square, and eventually a rhombic crystal lattice (flat). According to the findings, the water undergoes a series of transitions known as “triple points,” when all three phases coexist.
Not solid or liquid but hexatic
The water transformed into a previously unobserved exotic state at ambient temperature and the pressure of 0.5 to 2 gigapascals characteristic of nanoscale channels. Neither a solid nor a liquid, the water had taken on a new phase. Water molecules still have their atoms grouped approximately in a hexagonal configuration, but they are constantly spinning in a circle in this newly discovered phase.
Some theoretical physicists have speculated about such a condition of water, but until now it had never been seen. The “hexatic” phase is so named because of its unusual mix of hexagonal symmetry and high rotation. Kapil’s team’s calculations, to the best of our knowledge, are the first to propose that this hexatic state already exists in nanochannels at ambient temperature and, hence, may be identified experimentally.
Superionic even at moderate pressure
Later in the study, the monolayer water altered its state once again, this time becoming superionic, at temperatures of roughly 167 degrees Fahrenheit (75 degrees Celsius) and pressures of four gigapascals. This superionic state is characterized by the oxygen atoms remaining in their localized positions and forming a lattice, while the hydrogen protons freely move through this lattice. This state increases the conductivity of water.
Incredible as it may seem, for ordinary, unconfined water to become superionic, it requires temperatures over 790 degrees Fahrenheit (420 degrees Celsius) and pressures of about 56 gigapascals, or around the same value as deep below the Earth.
Since superionic phases often only form in very high-pressure environments like the cores of Uranus and Neptune, their occurrence under circumstances that are relatively simple to manufacture is remarkable. Recent reports suggest that the substance making up Earth’s inner core may likewise have superionic properties.
The practical applications of superionic water
The research verifies how drastically the characteristics of materials change when they are compressed into a single or a few molecular layers. Hexatic water is one of the numerous unique phases of water. Because hexatic and superionic water exist naturally in small channels under rather typical circumstances.
Superionic water may have certain useful applications: When water is in its superionic phase, it exhibits conductivity that is 100–1,000 times greater than that of typical battery electrolytes. Since this condition may be created by applying a little amount of pressure in nanochannels or between graphene layers, this may pave the way for more efficient batteries. Given constrained circumstances, additional chemicals also become superionic sooner than previously anticipated.